 |
市場調查報告書
商品編碼
1771299
脂質CMO市場:產業趨勢及全球預測 - 依脂質類型、公司規模、業務規模和主要地區Lipid CMO Market: Industry Trends and Global Forecasts - Distribution by Type of Lipid, Company Size, Scale Of Operation and Key Geographical Regions |
||||||
全球脂質CMO市場:概覽
今年全球脂質CMO市場規模達24億美元。預計預測期內,市場年複合成長率將達到 10.8%。
市場區隔包括根據以下參數進行的市場規模和機會分析:
脂質類型
- 脂質體/脂質奈米粒
- 磷脂
- 聚乙二醇化脂質
- 可離子化脂質(陽離子/陰離子脂質)
- 三酸甘油酯
- 鞘脂
- 中性脂質
- 其他
公司規模
- 小型
- 中型
- 大型/超大型
企業規模
- 臨床前
- 臨床試驗
- 商業化
大型地區
- 北美
- 歐洲
- 亞太地區
- 中東和北非
- 拉丁美洲
- 世界其他地區
全球脂質CMO市場:成長與趨勢
目前,約90%的在研候選藥物和近40%的已核准藥物面臨與溶解度和滲透性相關的挑戰。因此,在現代監管標準下,許多有前景的療法由於生物利用度低而無法通過臨床試驗並進入市場。為了解決這些問題,生物製藥產業的創新者設計了各種策略來改善藥物化合物的理化性質和整體類藥行為。在這些方法中,脂質奈米顆粒和其他能夠提高生物膜滲透性的脂質基輔料引起了藥物開發者的濃厚興趣。事實上,基於mRNA的新型COVID-19疫苗已利用脂質奈米顆粒有效地將活性成分遞送至人體內的標靶抗原呈現細胞。
此外,許多公司利用基於脂質的解決方案來重新配製現有的候選藥物,以提高其生物利用度,這大幅推動了對脂質藥物載體和輔料的需求。然而,某些醫用脂質的生產,尤其是用於脂質體和脂質奈米顆粒製劑的生產,仍然高度複雜、資金密集且極具挑戰性。因此,越來越多的製藥公司將脂質的生產外包給專業機構。與醫療級脂質的合約生產組織(CMO)合作可帶來許多益處,包括獲得先進技術、擴大生產能力和提高營運靈活性。此外,預計在預測期內,對高品質脂質的需求不斷成長將顯著推動專業合約製造業的市場成長。
全球脂質CMO市場:關鍵洞察
本報告深入探討了全球脂質CMO市場的現狀,並識別了產業內的潛在成長機會。報告的主要調查結果包括:
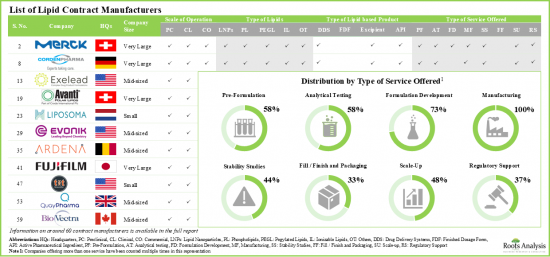
- 目前,全球約有60家公司聲稱提供合約製造服務,以協助開發和生產用於治療開發及其他相關產品的脂質。
- 該行業中的一些CMO具備為不同類型的脂質產品提供廣泛服務的必要能力,許多CMO擁有處理任何規模服務的能力。
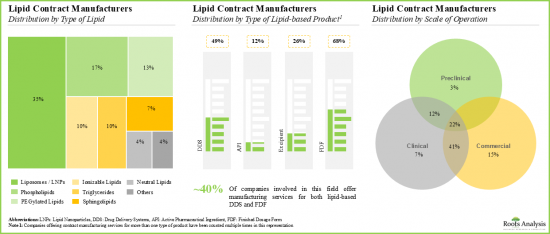
- 近35%提供脂質DDS合約製造服務的公司聲稱擁有商業規模生產脂質體/LNP所需的能力和專業知識。
- 自2010年以來成立的利害關係人中,近40%有能力提供臨床和商業規模的合約生產服務,其中約60%位於美國。
- 超大脂質體/LNP的代表性企業包括(依字母順序排列)AMRI、CordenPharma、Evonik、Fresenius Kabi、Fujifilm、Merck和TTY Biopharm。
- 為了獲得供應鏈能力並滿足申辦者不斷變化的需求,脂質CMO公司關鍵地區建立業務。
- 為了追求競爭優勢,企業投資或打算投資精力和資金來增強其現有的服務組合和合作產品。
- 最近,該行業的合作活動激增,其中大多數交易是為了從其他公司獲得獨特能力而簽署的。
- 由於全球對用於生產基於 mRNA 的COVID-19 候選疫苗的脂質奈米顆粒的需求激增,脂質 CMO 行業的交易量激增。
- 簽署的合作關係中,近 50%是製造協議。此外,約 65%的協議是為製造基於脂質的藥物遞送系統或輔料而簽署的。
- 近 75%的合作關係以北美為中心。與北美公司簽署協議的公司包括 ABITEC、AMRI、Catalent 和 Precision NanoSystems。
- 為了滿足日益成長的脂質需求,CMO/CDMO近年來進行了大規模擴張,其中大部分在美國設立了生產基地。
- 近年來,脂質製劑已成為提高藥物生物利用度的一種流行方法,近55家公司為此開發各種脂質相關技術。
- 該市場的供應端主要由中型和大型CMO驅動。值得注意的是,目前有近65%的產能位於北美生產工廠。
- 從中長期來看,預計脂質製劑開發商將繼續將生產營運外包給CMO/CDMO,使該服務市場以超過10.8%的年複合成長率成長。
脂質CMO市場參與者
- Avanti Polar Lipids
- Creative Biolabs
- Exelead
- FormuMax Scientific
- T&T Scientific
- Ardena
- Corden Pharma
- Evonik
- Fresenius Kabi
- Merck KGaA
- Fujifilm
- Nagase Medicals
- Nippon Fine Chemical
- TTY Biopharm
- VCARE Bio Labs
本報告調查全球脂質CMO市場提供市場概述,以及依脂質類型、公司規模、業務規模和地區的趨勢,和參與市場的公司簡介。
目錄
第1章 引言
第2章 執行摘要
第3章 導論
- 章節概述
- 脂質簡介
- 脂質在製藥業的應用
- 脂質製造面臨的挑戰
- 脂質製造外包的需求
- 結論
第4章 競爭格局
- 章節概述
- 脂質CMO參與者:市場格局
第5章 競爭格局分析
- 章節概述
- 假設和關鍵參數
- 研究方法
- 脂質CMO 廠商:競爭格局分析
第6章 公司簡介:北美脂質 CMO 廠商
- 章節概述
- Avanti Polar Lipids
- Creative Biolabs
- Exelead
- FormuMax Scientific
- T&T Scientific
第7章 公司簡介:歐洲脂質 CMO 廠商
- 章節概述
- Ardena
- CordenPharma
- Evonik
- Fresenius Kabi
- Merck KGaA
第8章 公司簡介:亞太脂質 CMO廠商
- 章節概述
- 富士軟片
- 長瀨醫療
- 日本精細化學
- TTY 生物製藥
- VCARE 生物實驗室
第9章 合作夥伴關係與合作
- 章節概述
- 合作模式
- 脂質CMO市場:合作夥伴關係與合作
第10章 近期擴張
第11章 產能分析
- 章節概述
- 關鍵假設與研究方法
- 脂質合約製造:全球裝置容量(百萬公升)
- 結論
第12章 自主或外購決策架構
- 章節概述
- 假設和參數定義
- 脂質合約製造:自主或外購決策
- 結論
第13章 市場預測與機會分析
- 章節概述
- 預測研究方法與關鍵假設
- 2035年全球脂質CMO市場
- 脂質CMO市場(2035年):依脂質類型
- 脂質CMO市場(2035年):依公司規模
- 脂質CMO市場(2035年):依公司規模
- 脂質CMO市場(2035年):依公司規模
- 脂質CMO市場(2035年):依區域
第14章 案例研究:脂質及脂質製劑技術的應用
- 章節概述
- 生物藥學分類系統
- 脂質製劑作為藥物傳遞系統
- 脂質製劑技術開發者
第15章 結論
第16章 高層洞察
第17章 附錄1:表格資料
第18章 附錄2:公司與組織清單
GLOBAL LIPID CMO MARKET: OVERVIEW
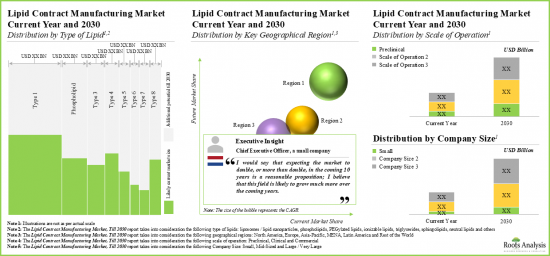
As per Roots Analysis, the global lipid CMO market valued at USD 2.4 billion in the current year is expected to grow at a CAGR of 10.8% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Lipid
- Liposomes / Lipid Nanoparticles
- Phospholipids
- Pegylated Lipids
- Ionizable Lipids (Cationic / Anionic Lipids)
- Triglycerides
- Sphingolipids
- Neutral Lipids
- Others
Company Size
- Small
- Mid-Sized
- Large / Very Large
Scale Of Operation
- Preclinical
- Clinical
- Commercial
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
- Latin America
- Rest of the World
GLOBAL LIPID CMO MARKET: GROWTH AND TRENDS
Currently, ~ 90% of drug candidates in the development stage and nearly 40% of approved pharmaceutical products face challenges related to solubility and permeability. As a result, under modern regulatory standards, many promising therapeutic leads fail to progress through clinical trials and reach the market due to poor bioavailability. To address these issues, innovators in the biopharmaceutical industry have devised various strategies to enhance the physicochemical properties and overall drug-like behavior of pharmaceutical compounds. Among these approaches, lipid nanoparticles and other lipid-based excipients, which improve permeability across biological membranes have attracted significant interest from drug developers. In fact, the novel mRNA-based COVID-19 vaccines utilized lipid nanoparticles to effectively deliver their active ingredients to target antigen-presenting cells within the human body.

Furthermore, numerous companies are leveraging lipid-based solutions to reformulate existing drug candidates and enhance their bioavailability, driving significant growth in the demand for lipid drug carriers and excipients. However, manufacturing certain medically relevant lipids, particularly those used in liposome and lipid nanoparticle formulations remains highly complex, capital-intensive, and challenging. Consequently, an increasing number of pharmaceutical companies are opting to outsource lipid manufacturing to specialized providers. Partnering with contract manufacturing organizations (CMOs) for medical-grade lipids offers several advantages, including access to advanced technologies, greater production capacity and enhanced operational flexibility. Moreover, we anticipate that the growing demand for high-quality lipids will significantly propel market growth in the specialty contract manufacturing sector over the forecast period.
GLOBAL LIPID CMO MARKET: KEY INSIGHTS
The report delves into the current state of global lipid CMO market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, around 60 players, across the world, claim to provide contract manufacturing services to support the development and production of lipids for use in therapeutic development, as well as other associated products.
- Several CMOs engaged in this industry have the required capabilities to offer a range of services for different types of lipid-based products; many CMOs have the capacity to operate across all scales.
- Close to 35% of players offering contract manufacturing services for lipid-based DDS claim to have the required capabilities and expertise to manufacture liposomes / LNPs at the commercial scale.

- Nearly 40% of the stakeholders established post-2010 have the capability to offer contract manufacturing services at both clinical and commercial scales; around 60% of these players are based in the US.
- Prominent examples of very large players offering liposomes / LNPs include (in alphabetical order) AMRI, CordenPharma, Evonik, Fresenius Kabi, Fujifilm, Merck and TTY Biopharm.
- In order to acquire supply chain competencies and meet the evolving needs of sponsors, lipid contract manufacturers have established a presence in key geographical regions.
- In pursuit of competitive edge, companies have made / revealed intentions to make investments, in terms of both effort and capital, to augment their existing service portfolios and affiliated offerings.
- Recently, there has been a surge in partnership activity within this industry; most deals were inked for the purpose of acquiring the proprietary capabilities of other players
- The lipid CMO industry witnessed a surge in the number of deals owing to the sudden increase in demand for lipid nanoparticles for the production of mRNA-based COVID-19 vaccine candidates, globally.
- Close to 50% of the partnerships inked were manufacturing agreements. Further, around 65% of these agreements were signed for the production of either lipid-based drug delivery systems or excipients.
- Nearly 75% of the partnership activity is centered in North America. Examples of firms that have signed deals within players based in North America, include ABITEC, AMRI, Catalent and Precision NanoSystems.
- To keep pace with the growing demand for lipids, CMOs / CDMOs have undertaken a number of expansion initiatives in the recent past; the maximum number of such instances involved facilities based in the US.
- Lipid-based formulations have emerged as a popular approach for improving drug bioavailability over the past years; close to 55 companies have developed various lipid-related technologies for this purpose.
- The supply side of this market is primarily driven by mid-to-large sized CMOs; interestingly, close to 65% of the currently available capacity is installed in production plants located in North America.

- In the mid-long term, we expect lipid-based formulation developers to continue outsourcing their manufacturing operations to CMOs / CDMOs, thereby, enabling this services market to grow at a CAGR of more than 10.8%.
Example Players in the Lipid CMO Market
- Avanti Polar Lipids
- Creative Biolabs
- Exelead
- FormuMax Scientific
- T&T Scientific
- Ardena
- Corden Pharma
- Evonik
- Fresenius Kabi
- Merck KGaA
- Fujifilm
- Nagase Medicals
- Nippon Fine Chemical
- TTY Biopharm
- VCARE Bio Labs
PRIMARY RESEARCH OVERVIEW
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews conducted with the following industry stakeholders:
- Founder and Chief Executive Officer, Company A
- Associate Director, Formulation Development, Company B
- Acting Manager, Biotech Process Development and Director, Process Development - Chemistry, Company C
GLOBAL LIPID CMO MARKET
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global lipid CMO market, focusing on key market segments, including [A] type of lipid, [B] company size, [C] scale of operation and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies offering contract services for the manufacturing of lipids, considering various parameters, such as [A] year of establishment, [B] company size, [C] scale of operation, [D] location of headquarters, [E] location of manufacturing facilities, [F] type of product, [G] type of service(s) offered and [H] type of lipid manufactured.
- Company Competitiveness Analysis: An insightful competitive analysis of the lipid manufacturers, examining factors, such as [A] supplier power and [B] service strength.
- Company Profiles: In-depth profiles of companies that offer various lipid manufacturing services, across North America, Europe and Asia-Pacific, focusing on [A] company overview, [B] financial performance (if available), [C] service portfolio and [D] recent developments and an informed future outlook.
- Partnerships and Collaborations: An in-depth analysis of the deals inked by stakeholders in this domain, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of focus area, [D] most active players (in terms of the number of partnerships signed) and [E] geographical distribution of partnership activity.
- Recent Expansions: A detailed analysis of the recent expansions, based on several relevant parameters, including [A] year of expansion, [B] type of expansion, [C] scale of operation, [D] type of product, [E] amount invested, [F] company size, [G] location of headquarters, [H] geographical location of the expanded facility, [I] most active players and [J] geographical distribution of the expansion activity.
- Capacity Analysis: A comprehensive analysis of global installed capacity for lipids, based on several relevant parameters, such as [A] company size, [B] scale of operation and [C] key geographical regions.
- Make Versus Buy Decision Making Framework: A detailed analysis, highlighting the various factors that need to be taken into consideration by lipid developers while deciding whether to manufacture their respective products in-house or engage the services of a CMO.
- Case study: An elaborate discussion on the wide adoption of lipids as drug delivery systems in mRNA vaccines.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to Lipids
- 3.2.1. Types of Lipids
- 3.3. Applications of Lipids in Pharmaceutical Industry
- 3.4. Challenges Associated with Lipid Manufacturing
- 3.5. Need for Outsourcing Lipid Manufacturing
- 3.6. Concluding Remarks
4. COMPETITIVE LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Lipid Contract Manufacturers: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Scale of Operation
- 4.2.4. Analysis by Location of Headquarters
- 4.2.5. Analysis by Location of Lipid Manufacturing Facilities
- 4.2.6. Analysis by Type of Product
- 4.2.7. Analysis by Service(s) Offered
- 4.2.8. Analysis by Types of Lipids Manufactured
- 4.2.8.1. Analysis by Types of Lipids Manufactured and Company Size
- 4.2.8.2. Analysis by Types of Lipids Manufactured and Scale of Operation
- 4.2.8.3. Analysis by Types of Lipids Manufactured and Location of Headquarters
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Lipid Contract Manufacturers: Company Competitiveness Analysis
- 5.4.1. Company Competitiveness Analysis: Lipid Contract Manufacturers in North America
- 5.4.2. Company Competitiveness Analysis: Lipid Contract Manufacturers in Europe
- 5.4.3. Company Competitiveness Analysis: Lipid Contract Manufacturers in Asia-Pacific
6. COMPANY PROFILES: LIPID CONTRACT MANUFACTURERS IN NORTH AMERICA
- 6.1. Chapter Overview
- 6.2. Avanti Polar Lipids
- 6.2.1. Company Overview
- 6.2.2. Lipid Manufacturing Service Offerings
- 6.2.3. Manufacturing Facilities
- 6.2.4. Recent Developments and Future Outlook
- 6.3. Creative Biolabs
- 6.3.1. Company Overview
- 6.3.2. Lipid Manufacturing Service Offerings
- 6.3.3. Manufacturing Facilities
- 6.3.4. Recent Developments and Future Outlook
- 6.4. Exelead
- 6.4.1. Company Overview
- 6.4.2. Lipid Manufacturing Service Offerings
- 6.4.3. Manufacturing Facilities
- 6.4.4. Recent Developments and Future Outlook
- 6.5. FormuMax Scientific
- 6.5.1. Company Overview
- 6.5.2. Lipid Manufacturing Service Offerings
- 6.5.3. Manufacturing Facilities
- 6.5.4. Recent Developments and Future Outlook
- 6.6. T&T Scientific
- 6.6.1. Company Overview
- 6.6.2. Lipid Manufacturing Service Offerings
- 6.6.3. Manufacturing Facilities
- 6.6.4. Recent Developments and Future Outlook
7. COMPANY PROFILES: LIPID CONTRACT MANUFACTURERS IN EUROPE
- 7.1. Chapter Overview
- 7.2. Ardena
- 7.2.1. Company Overview
- 7.2.2. Lipid Manufacturing Service Offerings
- 7.2.3. Manufacturing Facilities
- 7.2.4. Recent Developments and Future Outlook
- 7.3. CordenPharma
- 7.3.1. Company Overview
- 7.3.2. Lipid Manufacturing Service Offerings
- 7.3.3. Manufacturing Facilities
- 7.3.4. Recent Developments and Future Outlook
- 7.4. Evonik
- 7.4.1. Company Overview
- 7.4.2. Lipid Manufacturing Service Offerings
- 7.4.3. Manufacturing Facilities
- 7.4.4. Recent Developments and Future Outlook
- 7.5. Fresenius Kabi
- 7.5.1. Company Overview
- 7.5.2. Lipid Manufacturing Service Offerings
- 7.5.3. Manufacturing Facilities
- 7.5.4. Recent Developments and Future Outlook
- 7.6. Merck KGaA
- 7.6.1. Company Overview
- 7.6.2. Lipid Manufacturing Service Offerings
- 7.6.3. Manufacturing Facilities
- 7.6.4. Recent Developments and Future Outlook
8. COMPANY PROFILES: LIPID CONTRACT MANUFACTURERS IN ASIA-PACIFIC
- 8.1. Chapter Overview
- 8.2. Fujifilm
- 8.2.1. Company Overview
- 8.2.2. Lipid Manufacturing Service Offerings
- 8.2.3. Manufacturing Facilities
- 8.2.4. Recent Developments and Future Outlook
- 8.3. Nagase Medicals
- 8.3.1. Company Overview
- 8.3.2. Lipid Manufacturing Service Offerings
- 8.3.3. Manufacturing Facilities
- 8.3.4. Recent Developments and Future Outlook
- 8.4. Nippon Fine Chemical
- 8.4.1. Company Overview
- 8.4.2. Lipid Manufacturing Service Offerings
- 8.4.3. Manufacturing Facilities
- 8.4.4. Recent Developments and Future Outlook
- 8.5. TTY Biopharm
- 8.5.1. Company Overview
- 8.5.2. Lipid Manufacturing Service Offerings
- 8.5.3. Manufacturing Facilities
- 8.5.4. Recent Developments and Future Outlook
- 8.6. VCARE Bio Labs
- 8.6.1. Company Overview
- 8.6.2. Lipid Manufacturing Service Offerings
- 8.6.3. Manufacturing Facilities
- 8.6.4. Recent Developments and Future Outlook
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Lipid Contract Manufacturing Market: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Type of Product
- 9.3.4. Analysis by Type of Focus Area
- 9.3.5. Most Active Players: Analysis by Number of Partnerships
- 9.3.6. Geographical Analysis
- 9.3.6.1. Region-wise Distribution
- 9.3.6.2. Country-wise Distribution
10. RECENT EXPANSIONS
- 10.1. Chapter Overview
- 10.2. Lipid Contract Manufacturing Market: Recent Expansions
- 10.2.1. Analysis by Year of Expansion
- 10.2.2. Analysis by Type of Expansion
- 10.2.3. Analysis by Type of Expansion and Scale of Operation
- 10.2.4. Analysis by Type of Product
- 10.2.5. Analysis by Amount Invested
- 10.2.6. Analysis by Company Size and Location of Headquarters
- 10.2.7. Analysis by Location of Expanded Facility
- 10.2.8. Most Active Players: Analysis by Number of Recent Expansions
- 10.2.9. Geographical Analysis
- 10.2.9.1. Region-wise Distribution
- 10.2.9.2. Country-wise Distribution
11. CAPACITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Key Assumptions and Methodology
- 11.3. Lipid Contract Manufacturing: Installed Global Capacity (Million Liters)
- 11.3.1. Analysis by Company Size
- 11.3.2. Analysis by Scale of Operation
- 11.3.3. Analysis by Location of Manufacturing Facility
- 11.4. Concluding Remarks
12. MAKE VERSUS BUY DECISION MAKING FRAMEWORK
- 12.1. Chapter Overview
- 12.2. Assumptions and Parameter Definitions
- 12.3. Lipid Contract Manufacturing: Make Versus Buy Decision Making
- 12.3.1. Scenario 1
- 12.3.2. Scenario 2
- 12.3.3. Scenario 3
- 12.3.4. Scenario 4
- 12.4. Concluding Remarks
13. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 13.1. Chapter Overview
- 13.2. Forecast Methodology and Key Assumptions
- 13.3. Global Lipid Contract Manufacturing Market, Till 2035
- 13.4. Lipid Contract Manufacturing Market, Till 2035: Distribution by Type of Lipid
- 13.5. Lipid Contract Manufacturing Market, Till 2035: Distribution by Company Size
- 13.6. Lipid Contract Manufacturing Market, Till 2035: Distribution by Scale of Operation
- 13.7. Lipid Contract Manufacturing Market, Till 2035: Distribution by Region
- 13.7.1. Lipid Contract Manufacturing Market in North America, Till 2035
- 13.7.2. Lipid Contract Manufacturing Market in Europe, Till 2035
- 13.7.3. Lipid Contract Manufacturing Market in Asia-Pacific, Till 2035
- 13.7.4. Lipid Contract Manufacturing Market in MENA, Till 2035
- 13.7.5. Lipid Contract Manufacturing Market in Latin America, Till 2035
- 13.7.6. Lipid Contract Manufacturing Market in Rest of the World, Till 2035
14. CASE STUDY: APPLICATIONS OF LIPIDS AND LIPID FORMULATION TECHNOLOGIES
- 14.1. Chapter Overview
- 14.2. Biopharmaceutical Drug Classification System
- 14.3. Lipid-based Formulations as Drug Delivery Systems
- 14.3.1. Role of Lipids in mRNA Vaccines
- 14.3.2. Recent Developments in Lipid Nanoparticles
- 14.4. Lipid-based Formulation Technology Developers
- 14.4.1. Analysis by Year of Establishment
- 14.4.2. Analysis by Company Size
- 14.4.3. Analysis by Location of Headquarters
15. CONCLUDING REMARKS
16. EXECUTIVE INSIGHTS
- 16.1. Chapter Overview
- 16.2. Company A
- 16.2.1. Company Snapshot
- 16.2.2. Interview Transcript: Founder and CEO
- 16.3. Company B
- 16.3.1. Company Snapshot
- 16.3.2. Interview Transcript: Associate Director, Formulation Development
- 16.4. Company C
- 16.4.1. Company Snapshot
- 16.4.2. Interview Transcript: Acting Manager, Biotech Process Development and Director, Process Development - Chemistry
17. APPENDIX 1: TABULATED DATA
18. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 4.1 List of Lipid Contract Manufacturers
- Table 4.2 Lipid Contract Manufacturers: Information on Number and Location of Manufacturing Facilities
- Table 4.3 Lipid Contract Manufacturers: Information on Type of Product and Scale of Operation
- Table 4.4 Lipid Contract Manufacturers: Information on Service(s) Offered
- Table 4.5 Lipid Contract Manufacturers: Information on Types of Lipids Manufactured
- Table 6.1 Lipid Contract Manufacturers: List of Profiled Companies (North America)
- Table 6.2 Avanti Polar Lipids: Company Snapshot
- Table 6.3 Avanti Polar Lipids: Lipid-related Service Offerings
- Table 6.4 Avanti Polar Lipids: Information on Manufacturing Facility
- Table 6.5 Avanti Polar Lipids: Recent Developments and Future Outlook
- Table 6.6 Creative Biolabs: Company Snapshot
- Table 6.7 Creative Biolabs: Lipid-related Service Offerings
- Table 6.8 Creative Biolabs: Information on Manufacturing Facility
- Table 6.9 Exelead: Company Snapshot
- Table 6.10 Exelead: Lipid-related Service Offerings
- Table 6.11 Exelead: Information on Manufacturing Facility
- Table 6.12 Exelead: Recent Developments and Future Outlook
- Table 6.13 FormuMax Scientific: Company Snapshot
- Table 6.14 FormuMax Scientific: Lipid-related Service Offerings
- Table 6.15 FormuMax Scientific: Information on Manufacturing Facility
- Table 6.16 T&T Scientific: Company Snapshot
- Table 6.17 T&T Scientific: Lipid-related Service Offerings
- Table 6.18 T&T Scientific: Information on Manufacturing Facility
- Table 6.19 T&T Scientific: Recent Developments and Future Outlook
- Table 7.1 Lipid Contract Manufacturers: List of Profiled Companies (Europe)
- Table 7.2 Ardena: Company Snapshot
- Table 7.3 Ardena: Lipid-related Service Offerings
- Table 7.4 Ardena: Information on Manufacturing Facility
- Table 7.5 Ardena: Recent Developments and Future Outlook
- Table 7.6 CordenPharma: Company Snapshot
- Table 7.7 CordenPharma: Lipid-related Service Offerings
- Table 7.8 CordenPharma: Information on Manufacturing Facility
- Table 7.9 CordenPharma: Recent Developments and Future Outlook
- Table 7.10 Evonik: Company Snapshot
- Table 7.11 Evonik: Lipid-related Service Offerings
- Table 7.12 Evonik: Information on Manufacturing Facility
- Table 7.13 Evonik: Recent Developments and Future Outlook
- Table 7.14 Fresenius Kabi: Company Snapshot
- Table 7.15 Fresenius Kabi: Lipid-related Service Offerings
- Table 7.16 Fresenius Kabi: Information on Manufacturing Facility
- Table 7.17 Fresenius Kabi: Recent Developments and Future Outlook
- Table 7.18 Merck KGaA: Company Snapshot
- Table 7.19 Merck KGaA: Lipid-related Service Offerings
- Table 7.20 Merck KGaA: Information on Manufacturing Facility
- Table 7.21 Merck KGaA: Recent Developments and Future Outlook
- Table 8.1 Lipid Contract Manufacturers: List of Profiled Companies (Asia-Pacific)
- Table 8.2 Fujifilm: Company Snapshot
- Table 8.3 Fujifilm: Lipid-related Service Offerings
- Table 8.4 Fujifilm: Information on Manufacturing Facility
- Table 8.5 Fujifilm: Recent Developments and Future Outlook
- Table 8.6 Nagase Medicals: Company Snapshot
- Table 8.7 Nagase Medicals: Lipid-related Service Offerings
- Table 8.8 Nagase Medicals: Information on Manufacturing Facility
- Table 8.9 Nagase Medicals: Recent Developments and Future Outlook
- Table 8.10 Nippon Fine Chemical: Company Snapshot
- Table 8.11 Nippon Fine Chemical: Lipid-related Service Offerings
- Table 8.12 Nippon Fine Chemical: Information on Manufacturing Facility
- Table 8.13 TTY Biopharm: Company Snapshot
- Table 8.14 TTY Biopharm: Lipid-related Service Offerings
- Table 8.15 TTY Biopharm: Information on Manufacturing Facility
- Table 8.16 TTY Biopharm: Recent Developments and Future Outlook
- Table 8.17 VCARE Bio Labs: Company Snapshot
- Table 8.18 VCARE Bio Labs: Lipid-related Service Offerings
- Table 9.1 Lipid Contract Manufacturers: List of Partnerships and Collaborations, Since 2016
- Table 10.1 Lipid Contract Manufacturers: Recent Expansions, Since 2016
- Table 14.1 Lipid Formulation Technologies: List of Developers
- Table 14.2 List of Approved Lipid-based Drugs
- Table 17.1 Lipid Contract Manufacturers: Distribution by Year of Establishment
- Table 17.2 Lipid Contract Manufacturers: Distribution by Company Size
- Table 17.3 Lipid Contract Manufacturers: Distribution by Scale of Operation
- Table 17.4 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Region-wise)
- Table 17.5 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Country-wise)
- Table 17.6 Lipid Contract Manufacturers: Distribution by Company Size and Location of Headquarters
- Table 17.7 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Region-wise)
- Table 17.8 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Country-wise)
- Table 17.9 Lipid Contract Manufacturers: Distribution by Type of Product
- Table 17.10 Lipid Contract Manufacturers: Distribution by Service(s) Offered
- Table 17.11 Lipid Contract Manufacturers: Distribution by Type of Product and Service(s) Offered
- Table 17.12 Lipid Contract Manufacturers: Distribution by Service(s) Offered and Location of Headquarters
- Table 17.13 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured
- Table 17.14 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Company Size
- Table 17.15 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Scale of Operation
- Table 17.16 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Location of Headquarters
- Table 17.17 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Table 17.18 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 17.19 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Table 17.20 Partnerships and Collaborations: Distribution by Type of Product
- Table 17.21 Partnerships and Collaborations: Year-wise Trend by Type of Product, Since 2016
- Table 17.22 Partnerships and Collaborations: Distribution by Type of Focus Area
- Table 17.23 Most Active Players: Distribution by Number of Partnerships
- Table 17.24 Partnerships and Collaborations: Distribution by Region (Continent-wise)
- Table 17.25 Partnerships and Collaborations: Distribution by Region (Country-wise)
- Table 17.26 Recent Expansions: Cumulative Year-wise Trend, Since 2016
- Table 17.27 Recent Expansions: Distribution by Type of Expansion
- Table 17.28 Recent Expansions: Distribution by Type of Expansion and Scale of Operation
- Table 17.29 Recent Expansions: Distribution by Type of Product
- Table 17.30 Recent Expansions: Distribution by Type of Expansion and Type of Product
- Table 17.31 Recent Expansions: Distribution by Amount Invested (USD Million)
- Table 17.32 Recent Expansions: Distribution by Company Size and Location of Headquarters
- Table 17.33 Recent Expansions: Distribution by Location of Expanded Facility
- Table 17.34 Recent Expansions: Distribution by Type of Expansion and Location of Expanded Facility
- Table 17.35 Most Active Players: Distribution by Number of Recent Expansions
- Table 17.36 Recent Expansions: Distribution by Year of Expansion and Region
- Table 17.37 Recent Expansions: Distribution by Region (Country-wise)
- Table 17.38 Lipid Contract Manufacturing Capacity: Distribution by Company Size
- Table 17.39 Lipid Contract Manufacturing Capacity: Distribution by Scale of Operation
- Table 17.40 Lipid Contract Manufacturing Capacity: Distribution by Location of Manufacturing Facility
- Table 17.41 Global Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.42 Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035: Distribution by Type of Product (USD Billion)
- Table 17.43 Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035: Distribution by Company Size (USD Billion)
- Table 17.44 Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035: Distribution by Scale of Operation (USD Billion)
- Table 17.45 Lipid Contract Manufacturing Market in North America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.46 Lipid Contract Manufacturing Market in Europe, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.47 Lipid Contract Manufacturing Market in Asia-Pacific, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.48 Lipid Contract Manufacturing Market in MENA, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.49 Lipid Contract Manufacturing Market in Latin America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.50 Lipid Contract Manufacturing Market in Rest of the World, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
List of Figures
- Figure 3.1 Applications of Lipid-based Formulations
- Figure 3.2 Classification of Lipids
- Figure 4.1 Lipid Contract Manufacturers: Distribution by Year of Establishment
- Figure 4.2 Lipid Contract Manufacturers: Distribution by Company Size
- Figure 4.3 Lipid Contract Manufacturers: Distribution by Scale of Operation
- Figure 4.4 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Region-wise)
- Figure 4.5 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Country-wise)
- Figure 4.6 Lipid Contract Manufacturers: Distribution by Company Size and Location of Headquarters
- Figure 4.7 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Region-wise)
- Figure 4.8 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Country-wise)
- Figure 4.9 Lipid Contract Manufacturers: Distribution by Type of Product
- Figure 4.10 Lipid Contract Manufacturers: Distribution by Service(s) Offered
- Figure 4.11 Lipid Contract Manufacturers: Distribution by Type of Product and Service(s) Offered
- Figure 4.12 Lipid Contract Manufacturers: Distribution by Service(s) Offered and Location of Headquarters
- Figure 4.13 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured
- Figure 4.14 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Company Size
- Figure 4.15 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Scale of Operation
- Figure 4.16 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Location of Headquarters
- Figure 5.1 Company Competitiveness Analysis: Lipid Contract Manufacturers in North America
- Figure 5.2 Company Competitiveness Analysis: Lipid Contract Manufacturers in Europe
- Figure 5.3 Company Competitiveness Analysis: Lipid Contract Manufacturers in Asia-Pacific
- Figure 9.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Figure 9.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 9.3 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Figure 9.4 Partnerships and Collaborations: Distribution by Type of Product
- Figure 9.5 Partnerships and Collaborations: Year-wise Trend by Type of Product, Since 2016
- Figure 9.6 Partnerships and Collaborations: Distribution by Type of Focus Area
- Figure 9.7 Most Active Players: Distribution by Number of Partnerships
- Figure 9.8 Partnerships and Collaborations: Distribution by Region (Continent-wise)
- Figure 9.9 Partnerships and Collaborations: Distribution by Region (Country-wise)
- Figure 10.1 Recent Expansions: Cumulative Year-wise Trend, Since 2016
- Figure 10.2 Recent Expansions: Distribution by Type of Expansion
- Figure 10.3 Recent Expansions: Distribution by Type of Expansion and Scale of Operation
- Figure 10.4 Recent Expansions: Distribution by Type of Product
- Figure 10.5 Recent Expansions: Distribution by Type of Expansion and Type of Product
- Figure 10.6 Recent Expansions: Distribution by Amount Invested (USD Million)
- Figure 10.7 Recent Expansions: Distribution by Company Size and Location of Headquarters
- Figure 10.8 Recent Expansions: Distribution by Location of Expanded Facility
- Figure 10.9 Recent Expansions: Distribution by Type of Expansion and Location of Expanded Facility
- Figure 10.10 Most Active Players: Distribution by Number of Recent Expansions
- Figure 10.11 Recent Expansions: Distribution by Year of Expansion and Region
- Figure 10.12 Recent Expansions: Distribution by Region (Country-wise)
- Figure 11.1. Lipid Contract Manufacturing Capacity: Distribution by Company Size
- Figure 11.2. Lipid Contract Manufacturing Capacity: Distribution by Scale of Operation
- Figure 11.3. Lipid Contract Manufacturing Capacity: Distribution by Location of Manufacturing Facility
- Figure 12.1. Make versus Buy Decision Making Framework
- Figure 12.2. Make versus Buy Decision Making: Description of Possible Scenarios
- Figure 13.1 Global Lipid Contract Manufacturing Market, Till 2035 (USD Billion)
- Figure 13.2 Lipid Contract Manufacturing Market, Till 2035: Distribution by Type of Product (USD Billion)
- Figure 13.3 Lipid Contract Manufacturing Market, Till 2035: Distribution by Company Size (USD Billion)
- Figure 13.4 Lipid Contract Manufacturing Market, Till 2035: Distribution by Scale of Operation (USD Billion)
- Figure 13.5 Lipid Contract Manufacturing Market in North America, Till 2035 (USD Billion)
- Figure 13.6 Lipid Contract Manufacturing Market in Europe, Till 2035 (USD Billion)
- Figure 13.7 Lipid Contract Manufacturing Market in Asia-Pacific, Till 2035 (USD Billion)
- Figure 13.8 Lipid Contract Manufacturing Market in MENA, Till 2035 (USD Billion)
- Figure 13.9 Lipid Contract Manufacturing Market in Latin America, Till 2035 (USD Billion)
- Figure 13.10 Lipid Contract Manufacturing Market in Rest of the World, Till 2035 (USD Billion)
- Figure 14.1 Biopharmaceutics Classification System (BCS)
- Figure 14.2 Types of Lipid-based Drug Delivery Systems
- Figure 14.3 Lipid-based mRNA Vaccines: Mechanism of Action
- Figure 15.1 Concluding Remarks: Overall Market Landscape
- Figure 15.2 Concluding Remarks: Partnerships and Collaborations
- Figure 15.3 Concluding Remarks: Recent Expansions
- Figure 15.4 Concluding Remarks: Capacity Analysis
- Figure 15.5 Concluding Remarks: Market Forecast










