 |
市場調查報告書
商品編碼
1848304
澱粉衍生物:市佔率分析、產業趨勢、統計、成長預測(2025-2030)Starch Derivatives - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
預計到 2025 年澱粉衍生物市場規模將達到 459.3 億美元,到 2030 年將達到 555.2 億美元,複合年成長率為 3.86%。
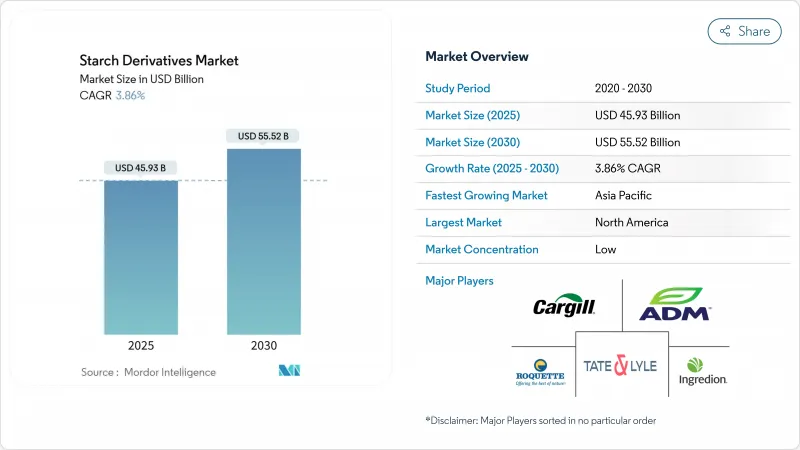
緩慢而穩定的擴張反映了從大宗商品到高價特種衍生品的轉變。監管機構目前更青睞植物來源原料,使得麥芽糊精、環糊精和葡萄糖漿在食品、飲料和藥品配方中的應用更加廣泛。酶法加工的投資持續成長,該技術可以減少能源消耗並簡化環境法規的合規性。北美供應商受益於成熟的食品藥物管理局(FDA) 框架,而亞太地區製造商則透過統一的安全標準和日益成長的膳食補充劑需求獲得了發展動力。在所有地區,潔淨標示和非基因定位已不再是可有可無的選擇,而已成為面向全球食品和藥品客戶的品牌原料供應商的關鍵競爭優勢。
全球澱粉衍生物市場趨勢與洞察
食品和飲料中對天然甜味劑的需求不斷成長
法律規範越來越青睞天然甜味劑而非合成替代品,例如 FDA 的公認安全 (GRAS) 稱號,簡化了澱粉基甜味劑系統的核准途徑。 FDA 將麥芽糊精歸類為無糖碳水化合物聚合物,其葡萄糖當量為 20 或更低,這使其在食品應用方面比合成替代品更具優勢。偏好向天然成分的轉變推動了整個飲料類別的改性舉措,對澱粉衍生的質構劑產生了持續的需求,這些質構劑既能保持口感,又能減少合成添加劑的含量。這種趨勢從食品和飲料延伸到機能性食品,在這些食品中,潔淨標示要求推動了天然衍生澱粉改質劑的使用,而不是化學加工的替代品。監管機構對成分透明度的重視加強了澱粉衍生物在需要天然成分標籤的應用中的競爭地位。
烘焙和糖果業對澱粉基葡萄糖漿的需求量很大
烘焙和糖果甜點應用充分利用了葡萄糖漿獨特的功能特性,尤其是其抑制結晶和延長保存期限的能力,同時又符合食品安全法規。聯合國糧農組織的《食品轉碼器》標準將葡萄糖漿認定為加工食品中不可或缺的功能性成分,支持其在全球市場的推廣。酵素法加工的創新使得葡萄糖漿的生產能夠調整葡萄糖當量值,使製造商能夠針對特定應用最佳化甜度和褐變特性,同時保持法規合規性。在高階糖果甜點領域,歐洲食品安全框架更傾向於使用天然葡萄糖漿而非合成替代品,這為市場差異化創造了機會。應用的技術複雜性和監管要求構成了進入壁壘,使得擁有核准配方的現有企業能夠擴展到鄰近類別,同時保持定價權。
農業原料成本波動影響產業盈利
原物料價格波動顯著影響澱粉衍生物的盈利,農業市場受天氣干擾和地緣政治緊張局勢的影響,進而影響全球供應鏈。根據美國農業部 (USDA) 的作物報告,主要澱粉產區的產量差異很大,直接影響下游加工商的原料供應和定價。玉米粉市場對農業政策和貿易限制的變化特別敏感,價格波動對綜合加工商的利潤帶來壓力。主要農業產區的天氣干擾迫使製造商維持高存量基準,增加了營運成本需求並降低了營運靈活性。政府的農業支持計畫和貿易政策進一步增加了原料價格的不確定性,需要採取複雜的對沖策略來管理成本波動。
細分分析
環糊精預計將成為一個快速成長的細分市場,到2030年複合年成長率將達到5.13%,這得益於FDA核准其用於醫藥領域,其獨特的分子結構能夠提高藥物溶解度並控制釋放製劑。 FDA已認可環糊精是藥物傳輸系統中的安全輔料,這支持了其在多個治療類別中的商業性潛力。麥芽糊精的市佔率在2024年將達到34.36%,這反映了其作為填充劑和風味載體的多功能性,並且在食品應用中已確立了GRAS地位。葡萄糖漿在烘焙食品領域保持穩定的需求,這得益於糧農組織食品轉碼器標準對其在加工食品中的功能性益處的認可。糊精受益於其在黏合劑和可生物分解包裝領域不斷擴大的工業應用,而環境法規也傾向於天然替代品。
近期,環糊精類藥物製劑已獲得監管部門核准,標誌著該領域已發展成為一個先進的藥物遞送平台,其應用範圍涵蓋多個治療領域。環糊精類藥物市場正日益體現出監管合規性要求,而非傳統的商品類別。由於技術差異化和成熟的核准途徑,特種衍生物的價格更高。改質環糊精因其增強的溶解性而獲得監管部門的認可,為特定用途衍生物在醫藥和營養保健品市場創造了機會。
木薯是成長最快的原料,到2030年複合年成長率將達到4.88%,這得益於其天然無麩質特性以及對全球市場潔淨標示法規的合規性。玉米憑藉其成熟的美國農業部 (USDA) 品質標準和完善的供應鏈基礎設施,確保始終如一的品質和合規性,到2024年將以63.22%的市場佔有率佔據市場主導地位。小麥衍生品在歐洲市場用於特殊應用,歐盟品質標準支持其在食品應用領域的高階定位。馬鈴薯澱粉在需要優異成膜性的應用中價格較高,其功能優勢也受到監管機構的認可。米澱粉在亞洲市場的重要性日益凸顯,當地食品安全法規支持其在傳統和現代食品領域的應用。
供應源多元化策略體現了監管風險管理的考量,企業需要持有多個供應商的核准,以確保供應鏈在監管變化的情況下仍能保持韌性。比較不同澱粉來源的監管狀況,為市場區隔創造了機會,有機和非基因改造認證能夠在注重健康的消費者群體中實現高階定位。農業投入品和加工方法的法律規範日益影響供應商的選擇決策,擁有全面合規計畫的供應商更受青睞。
區域分析
北美將在2024年維持市場領先地位,市佔率達36.23%。這得益於其全面的FDA法規結構,該框架為食品和藥品用澱粉衍生物的核准建立了清晰的路徑。該地區受益於美國農業部(美國)的農業品質標準,確保了原料的穩定供應,並建立了良好的生產規範,從而增強了出口競爭力。穩定的藥品應用監管為北美供應商帶來了競爭優勢,而FDA的核准途徑則使其在全球市場上佔據了優勢地位。該地區成熟的法規環境支持特種應用領域的創新,同時保持了確保消費者權益的安全標準。
亞太地區將成為成長最快的地區,到2030年複合年成長率將達到5.23%,這得益於東協市場監管協調舉措,旨在為澱粉衍生物創建標準化的核准途徑。區域食品安全框架日益與國際標準接軌,在確保產品品質和安全的同時,降低了跨國供應商的合規成本。該地區的成長反映了在認可國際品質標準的法律規範的支持下,製藥生產能力不斷增強。政府推動食品加工工業化的措施將創造對技術先進、符合不斷變化的安全要求的澱粉衍生物的需求。
歐洲正呈現穩定成長態勢,這得益於歐洲食品安全局(EFSA)的全面安全評估,該評估為食品和製藥業的澱粉衍生物應用制定了明確的指導方針。該地區嚴格的法律規範設置了准入門檻,同時透過經批准的產品系列和全面的合規計畫保護了現有供應商。歐盟環境法規更青睞可生物分解的澱粉基材料,而非石油基替代品,為永續包裝應用創造了市場機會。該地區對永續性的關注正在推動監管機構對符合循環經濟原則和環境保護標準的天然澱粉改質產品的支持。
其他福利:
- Excel 格式的市場預測 (ME) 表
- 3個月的分析師支持
目錄
第1章 引言
- 研究假設和市場定義
- 調查範圍
第2章調查方法
第3章執行摘要
第4章 市場狀況
- 市場概況
- 市場促進因素
- 食品和飲料中對天然甜味劑的需求不斷增加
- 烘焙和糖果甜點業對澱粉基葡萄糖漿的需求不斷增加
- 高果糖玉米糖漿 (HFCS) 在飲料配方中的應用日益增多
- 澱粉衍生物的多功能優勢
- 對潔淨標示和非基因改造成分的需求不斷成長
- 澱粉酶處理技術的進步。
- 市場限制
- 農業原物料價格波動影響產業盈利
- 食用高果糖玉米糖漿帶來的健康問題
- 消費者越來越拒絕人工添加劑
- 各種澱粉添加劑的過敏風險及標籤要求
- 供應鏈分析
- 監理展望
- 波特五力模型
- 新進入者的威脅
- 買家/消費者的議價能力
- 供應商的議價能力
- 替代品的威脅
- 競爭對手之間的競爭
第5章市場規模及成長預測
- 按類型
- 葡萄糖漿
- 高果糖玉米糖漿(HFCS)
- 麥芽糊精
- 環糊精
- 糊精
- 其他
- 按來源
- 玉米
- 小麥
- 馬鈴薯
- 木薯
- 其他
- 按形式
- 粉末
- 液體
- 按用途
- 食品/飲料
- 麵包店
- 糖果甜點
- 飲料
- 其他
- 製藥
- 個人護理和化妝品
- 動物飼料
- 其他
- 食品/飲料
- 按地區
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 其他北美地區
- 歐洲
- 德國
- 英國
- 義大利
- 法國
- 西班牙
- 荷蘭
- 其他歐洲地區
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 其他亞太地區
- 南美洲
- 巴西
- 阿根廷
- 南美洲其他地區
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
- 其他中東和非洲地區
- 北美洲
第6章 競爭態勢
- 市場集中度
- 策略趨勢
- 市場排名分析
- 公司簡介
- Archer Daniels Midland Company
- Cargill, Incorporated
- Ingredion Inc.
- Tate & Lyle Plc
- Roquette Freres SA
- Sudzucker AG
- Angel Starch & Food Pvt Ltd
- Tereos SA
- Royal Avebe
- Grain Processing Corp.
- Universal Starch Chem Allied Ltd.
- Gujarat Ambuja Exports Limited
- Thai Wah Public Co.
- Qingdao CBH Co.
- Matsutani Chemical Industry Co., Ltd.
- DSM-Firmenich
- Sunar Group
- Shandong Baolingbao
- Crespel & Deiters GmbH & Co. KG
- Bharat Starch
第7章 市場機會與未來展望
The starch derivatives market size is valued at USD 45.93 billion in 2025 and is forecast to reach USD 55.52 billion by 2030, advancing at a 3.86% CAGR.

Moderate but steady expansion reflects the transition from bulk commodities to specialty derivatives that command premium pricing. Regulatory agencies now favor plant-based inputs, enabling broader use of maltodextrin, cyclodextrin, and glucose syrups in food, beverage, and pharmaceutical formulations. Investment continues to move toward enzymatic processing that lowers energy use and simplifies compliance with environmental rules. North American suppliers benefit from a mature Food and Drug Administration (FDA) framework, while Asia-Pacific manufacturers gain momentum through harmonized safety standards and rising nutraceutical demand. Across all regions, clean-label and non-GMO positioning is no longer optional; it has become a key competitive lever for branded ingredient suppliers that target global food and drug customers.
Global Starch Derivatives Market Trends and Insights
Growing Demand for Natural Sweeteners in Food and Beverage
Regulatory frameworks increasingly favor naturally-derived sweeteners over synthetic alternatives, with the FDA's Generally Recognized as Safe (GRAS) designation streamlining approval pathways for starch-based sweetening systems. The FDA's classification of maltodextrin as a non-sweet saccharide polymer with dextrose equivalent below 20 positions it advantageously against synthetic alternatives in food applications. Consumer preference shifts toward natural ingredients drive reformulation initiatives across beverage categories, creating sustained demand for starch-derived texturizing agents that maintain mouthfeel while reducing synthetic additive content. The trend extends beyond beverages into functional foods, where clean-label requirements drive specification of naturally-derived starch modifications over chemically-processed alternatives. Regulatory bodies' emphasis on ingredient transparency strengthens the competitive position of starch derivatives in applications requiring natural ingredient declarations.
High Demand for Starch-Based Glucose Syrup in Bakery and Confectionary
Bakery and confectionery applications leverage glucose syrups' unique functional properties, particularly their ability to control crystallization and extend shelf life while maintaining compliance with food safety regulations. The FAO's Codex Alimentarius standards recognize glucose syrups as essential functional ingredients in processed foods, supporting their adoption across global markets. Enzymatic processing innovations enable glucose syrup production with tailored dextrose equivalent values, allowing manufacturers to optimize sweetness intensity and browning characteristics for specific applications while maintaining regulatory compliance. European food safety frameworks favor naturally-derived glucose syrups over synthetic alternatives in premium confectionery segments, creating market differentiation opportunities. The application's technical complexity and regulatory requirements create barriers to entry, enabling established players with approved formulations to maintain pricing power while expanding into adjacent categories.
Volatility in Agricultural Raw Material Costs Affect Industry Profitability
Raw material price volatility significantly impacts starch derivative profitability, with agricultural commodity markets subject to weather-related disruptions and geopolitical tensions affecting global supply chains. USDA crop reports indicate significant yield variations in key starch-producing regions, directly affecting raw material availability and pricing for downstream processors. Corn starch markets demonstrate particular sensitivity to agricultural policy changes and trade regulations, with price fluctuations creating margin pressure for integrated processors. Weather-related disruptions in key agricultural regions force manufacturers to maintain higher inventory levels, increasing working capital requirements and reducing operational flexibility. Government agricultural support programs and trade policies create additional uncertainty in raw material pricing, requiring sophisticated hedging strategies to manage cost volatility.
Other drivers and restraints analyzed in the detailed report include:
- Increased Adoption of High Fructose Corn Syrup (HFCS) in Beverage Formulation
- Amplifying Demand for Clean Label and Non-GMO Ingredients
- Rising Consumer Shift Away from Artificial Additives
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Cyclodextrin emerges as the fastest-growing segment with 5.13% CAGR through 2030, driven by FDA approvals for pharmaceutical applications where its unique molecular structure enables drug solubility enhancement and controlled release formulations. The FDA's recognition of cyclodextrins as safe excipients in drug delivery systems supports commercial viability across multiple therapeutic categories. Maltodextrin commands 34.36% market share in 2024, reflecting its versatility as a bulking agent and flavor carrier with established GRAS status across food applications. Glucose syrups maintain steady demand in bakery applications, supported by FAO Codex standards that recognize their functional benefits in processed foods. Dextrins benefit from expanding industrial uses in adhesives and biodegradable packaging, where environmental regulations favor naturally-derived alternatives.
Recent regulatory approvals for cyclodextrin-based pharmaceutical formulations demonstrate the segment's evolution toward sophisticated drug delivery platforms, with applications extending across multiple therapeutic areas. The type segmentation increasingly reflects regulatory compliance requirements rather than traditional commodity categories, with specialty derivatives commanding premium pricing through technical differentiation and established approval pathways. Modified cyclodextrins receive regulatory recognition for enhanced solubility properties, creating opportunities for application-specific derivatives in pharmaceutical and nutraceutical markets.
Tapioca represents the fastest-growing source at 4.88% CAGR through 2030, benefiting from its naturally gluten-free properties and compliance with clean-label regulations across global markets. Maize dominates with a 63.22% market share in 2024, supported by established USDA quality standards and a comprehensive supply chain infrastructure that ensures consistent quality and regulatory compliance. Wheat-based derivatives serve specialized applications in European markets, where EU quality standards support premium positioning in food applications. Potato starch commands premium pricing in applications requiring superior film-forming properties, supported by regulatory recognition of its functional benefits. Rice starch gains importance in Asian markets, where local food safety regulations support its use in traditional and modern food applications.
Source diversification strategies reflect regulatory risk management considerations, with companies maintaining multiple source approvals to ensure supply chain resilience despite regulatory changes. The comparative regulatory status of different starch sources creates market segmentation opportunities, with organic and non-GMO certifications enabling premium positioning in health-conscious consumer segments. Regulatory frameworks governing agricultural inputs and processing methods increasingly influence source selection decisions, favoring suppliers with comprehensive compliance programs.
The Starch Derivatives Market Report Segments the Industry Into Type (Glucose Syrup, High Fructose Corn Syrup, Maltodextrin, and More), Source (Maize, Wheat, Tapioca, Potato, and Other Sources), Form (Powder and Liquid), Application (Food and Beverage, Pharmaceutical Industry, and More), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and More). The Report Offers the Market Size in Terms of Value (USD).
Geography Analysis
North America maintains market leadership with 36.23% share in 2024, supported by comprehensive FDA regulatory frameworks that establish clear pathways for starch derivative approval across food and pharmaceutical applications. The region benefits from USDA agricultural quality standards that ensure consistent raw material supply and established good manufacturing practices that support export competitiveness. Regulatory stability in pharmaceutical applications creates competitive advantages for North American suppliers, with FDA approval pathways enabling premium positioning in global markets. The region's mature regulatory environment supports innovation in specialty applications while maintaining safety standards that ensure consumer protection.
Asia-Pacific emerges as the fastest-growing region with 5.23% CAGR through 2030, driven by regulatory harmonization initiatives across ASEAN markets that create standardized approval pathways for starch derivatives. Regional food safety frameworks increasingly align with international standards, reducing compliance costs for multinational suppliers while ensuring product quality and safety. The region's growth reflects expanding pharmaceutical manufacturing capabilities supported by regulatory frameworks that recognize international quality standards. Government initiatives promoting food processing industrialization create demand for technically sophisticated starch derivatives that comply with evolving safety requirements.
Europe demonstrates steady growth supported by comprehensive EFSA safety assessments that establish clear guidelines for starch derivative applications across food and pharmaceutical sectors. The region's stringent regulatory framework creates barriers to entry while protecting established suppliers with approved product portfolios and comprehensive compliance programs. EU environmental regulations favor biodegradable starch-based materials over petroleum-based alternatives, creating market opportunities for sustainable packaging applications. The region's focus on sustainability drives regulatory support for naturally-derived starch modifications that comply with circular economy principles and environmental protection standards.
- Archer Daniels Midland Company
- Cargill, Incorporated
- Ingredion Inc.
- Tate & Lyle Plc
- Roquette Freres S.A.
- Sudzucker AG
- Angel Starch & Food Pvt Ltd
- Tereos S.A.
- Royal Avebe
- Grain Processing Corp.
- Universal Starch Chem Allied Ltd.
- Gujarat Ambuja Exports Limited
- Thai Wah Public Co.
- Qingdao CBH Co.
- Matsutani Chemical Industry Co., Ltd.
- DSM-Firmenich
- Sunar Group
- Shandong Baolingbao
- Crespel & Deiters GmbH & Co. KG
- Bharat Starch
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Demand for Natural Sweeteners in Food and Beverage
- 4.2.2 High Demand for Starch-Based Glucose Syrup in Bakery and Confectionary
- 4.2.3 Increased Adoption of High Fructose Corn Syrup (HFCS) in Beverage Formulation
- 4.2.4 Multi-Functional Benefits Associated with Starch Derivatives
- 4.2.5 Amplifying Demand for Clean Label and Non-GMO Ingredients
- 4.2.6 Technological Advancements in Enzymatic Processing of Starch
- 4.3 Market Restraints
- 4.3.1 Volatility in Agricultural Raw Material Costs Affect Industry Profitability
- 4.3.2 Health Concerns Linked to High Fructose Corn Syrup Consumption
- 4.3.3 Rising Consumer Shift Away from Artificial Additives
- 4.3.4 Allergy Risks and Labeling Requirements for Various Starch Additives
- 4.4 Supply Chain Analysis
- 4.5 Regulatory Outlook
- 4.6 Porter's Five Forces
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers/Consumers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitute Products
- 4.6.5 Intensity of Competitive Rivalry
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Type
- 5.1.1 Glucose Syrups
- 5.1.2 High Fructose Corn Syrup (HFCS)
- 5.1.3 Maltodextrin
- 5.1.4 Cyclodextrin
- 5.1.5 Dextrins
- 5.1.6 Others
- 5.2 By Source
- 5.2.1 Maize
- 5.2.2 Wheat
- 5.2.3 Potato
- 5.2.4 Tapioca
- 5.2.5 Others
- 5.3 By Form
- 5.3.1 Powder
- 5.3.2 Liquid
- 5.4 By Application
- 5.4.1 Food and Beverage
- 5.4.1.1 Bakery
- 5.4.1.2 Confectionary
- 5.4.1.3 Beverage
- 5.4.1.4 Others
- 5.4.2 Pharmaceutial
- 5.4.3 Personal Care and Cosmetics
- 5.4.4 Animal Feed
- 5.4.5 Others
- 5.4.1 Food and Beverage
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.1.4 Rest of North America
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 Italy
- 5.5.2.4 France
- 5.5.2.5 Spain
- 5.5.2.6 Netherlands
- 5.5.2.7 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 India
- 5.5.3.3 Japan
- 5.5.3.4 Australia
- 5.5.3.5 Rest of Asia-Pacific
- 5.5.4 South America
- 5.5.4.1 Brazil
- 5.5.4.2 Argentina
- 5.5.4.3 Rest of South America
- 5.5.5 Middle East and Africa
- 5.5.5.1 South Africa
- 5.5.5.2 Saudi Arabia
- 5.5.5.3 United Arab Emirates
- 5.5.5.4 Rest of Middle East and Africa
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Ranking Analysis
- 6.4 Company Profiles (includes Global-level Overview, Market-level Overview, Core Segments, Financials (if available), Strategic Information, Market Rank/Share, Products & Services, Recent Developments)
- 6.4.1 Archer Daniels Midland Company
- 6.4.2 Cargill, Incorporated
- 6.4.3 Ingredion Inc.
- 6.4.4 Tate & Lyle Plc
- 6.4.5 Roquette Freres S.A.
- 6.4.6 Sudzucker AG
- 6.4.7 Angel Starch & Food Pvt Ltd
- 6.4.8 Tereos S.A.
- 6.4.9 Royal Avebe
- 6.4.10 Grain Processing Corp.
- 6.4.11 Universal Starch Chem Allied Ltd.
- 6.4.12 Gujarat Ambuja Exports Limited
- 6.4.13 Thai Wah Public Co.
- 6.4.14 Qingdao CBH Co.
- 6.4.15 Matsutani Chemical Industry Co., Ltd.
- 6.4.16 DSM-Firmenich
- 6.4.17 Sunar Group
- 6.4.18 Shandong Baolingbao
- 6.4.19 Crespel & Deiters GmbH & Co. KG
- 6.4.20 Bharat Starch












