 |
市場調查報告書
商品編碼
1740925
藥品防篡改包裝市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Pharmaceutical Tamper Proof Packaging Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
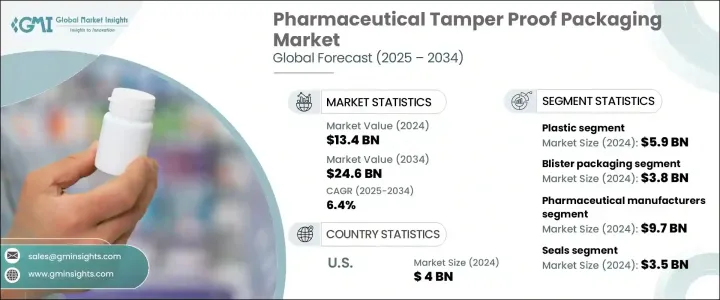
2024年,全球藥品防篡改包裝市場規模達134億美元,預計到2034年將以6.4%的複合年成長率成長,達到246億美元,這得益於全球藥品需求的激增和冷鏈物流規模的不斷擴大。隨著醫療保健服務越來越以患者為中心,藥品也變得越來越敏感,對能夠確保安全性和產品完整性的包裝的需求也比以往任何時候都更加強烈。藥品假冒事件的增多,加上監管審查的加強,正在推動整個供應鏈中對防篡改解決方案的需求。利害關係人正在快速創新,不僅要滿足法規合規性,還要滿足病患對更安全藥物的期望。全球藥品分銷日益複雜,尤其是隨著生物製劑、特殊療法和直銷模式的興起,這持續推動著對能夠確保產品在運輸過程中每個環節真實性的包裝形式的需求。此外,永續發展目標正在重塑製造商處理材料和設計的方式,使其同時專注於安全性和環境責任。
隨著藥品需求的不斷發展,防篡改包裝解決方案對於保護產品免受運輸過程中的污染、偽造和損壞至關重要。日益成長的監管壓力以及消費者對經過驗證的安全藥品的需求,正在推動材料和技術的創新。貿易政策的變化進一步影響了市場的成本結構,迫使製造商重新思考其採購模式。由於關稅和跨境限制加劇了財務和物流壓力,企業正在轉向近岸外包和回岸外包策略,以穩定供應鏈。這一趨勢可以縮短生產週期、加強品質控制,並更快地遵守區域安全法規。為了在日益嚴格的法規要求下保持領先地位,包裝供應商正在大力投資序列化技術、先進的防篡改功能以及基於區塊鏈的追蹤系統,以提高產品真實性並簡化監管審核,最終增強整個分銷鏈的信任。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 134億美元 |
| 預測值 | 246億美元 |
| 複合年成長率 | 6.4% |
2024年,塑膠材料領域引領藥品防篡改包裝市場,創造了59億美元的收入。塑膠因其在固體和液體藥物的瓶裝、泡殼包裝、封蓋和安瓿瓶方面的適應性而佔據主導地位。其輕量、成本效益高、易於大量生產的特性使其成為首選。同時,製造商擴大採用可生物分解和可回收的塑膠,例如PLA、PET和HDPE,以符合全球環保計劃和不斷發展的監管框架。
2024年,製藥業的銷售額達到97億美元,這得益於符合區域序列化和認證法規的防篡改包裝整合度的不斷提升。各大公司正利用配備追蹤系統和違規檢測功能的智慧包裝來強化供應鏈,尤其針對需要特殊處理方式的生物製劑和慢性病治療產品。
2024年,美國藥品防篡改包裝市場規模達40億美元。監管機構的執法力度加大,以及對病人安全的日益重視,推動了收縮帶、RFID封條和法醫標籤的廣泛應用。電子藥局和遠距病患照護模式的蓬勃發展,也推動了對包裝完整性的要求不斷提高,尤其是對溫度敏感和高風險藥物而言。
全球藥品防篡改包裝市場的主要參與者包括 NanoMatriX Technologies Limited、Applied DNA Sciences, Inc.、Lonza、Tamperguard、3M、SATO Holdings Corporation、WestRock Company、SICPA HOLDING SA、CCL Industries Inc.、SML Group、ACG、Authentix、Alien Technology Corp.、Avery Dennison Corporation、CordenVision、Cuthentix.各公司正在投資數位序列化、區塊鏈認證、可回收包裝和密封技術自動化,以保持競爭力。與製藥品牌合作客製化防篡改解決方案,並加大對永續材料的研發投入,正在塑造市場的未來。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 川普政府關稅
- 對貿易的影響
- 貿易量中斷
- 報復措施
- 對產業的影響
- 供給側影響(原料)
- 主要材料價格波動
- 供應鏈重組
- 生產成本影響
- 需求面影響(售價)
- 價格傳導至終端市場
- 市佔率動態
- 消費者反應模式
- 供給側影響(原料)
- 受影響的主要公司
- 策略產業反應
- 供應鏈重組
- 定價和產品策略
- 政策參與
- 展望與未來考慮
- 對貿易的影響
- 產業衝擊力
- 成長動力
- 全球藥品需求不斷成長
- 包裝技術的進步
- 醫藥電子商務擴張
- 增加研發投入
- 冷鏈物流擴張
- 產業陷阱與挑戰
- 實施成本高
- 環境問題
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按包裝類型,2021 - 2034 年
- 主要趨勢
- 吸塑包裝
- 條狀包裝
- 瓶子和罐子
- 小瓶和安瓿瓶
- 小袋和小袋
- 紙箱
- 注射器
- 其他
第6章:市場估計與預測:按防竄改技術,2021 - 2034 年
- 主要趨勢
- 感應封口
- 易碎瓶蓋/封蓋
- 薄膜包裝
- 泡殼箔
- 防篡改標籤和密封
- 電子追蹤系統
- 其他
第7章:市場估計與預測:按材料類型,2021 - 2034 年
- 主要趨勢
- 塑膠
- 寵物
- 高密度聚乙烯
- PVC
- 其他
- 玻璃
- 紙和紙板
- 鋁箔
- 其他
第8章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 製藥商
- 合約包裝組織(CPO)
第9章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- 3M
- ACG
- Alien Technology
- AlpVision
- Applied DNA Sciences
- Authentix
- Avery Dennison
- CCL Industries
- CordenPharma
- Essentra
- Lonza
- NanoMatrix Technologies
- SATO Holdings
- SICPA Holding
- SML Group
- Systech
- Tamperguard
- WestRock
The Global Pharmaceutical Tamper Proof Packaging Market was valued at USD 13.4 billion in 2024 and is estimated to grow at a CAGR of 6.4% to reach USD 24.6 billion by 2034, driven by surging global medicine demand and the expanding footprint of cold chain logistics. As healthcare delivery becomes more patient-centric and pharmaceutical products grow increasingly sensitive, the need for packaging that ensures security and product integrity is stronger than ever. Rising incidences of drug counterfeiting, coupled with heightened regulatory scrutiny, are creating a greater push for tamper-evident solutions across the supply chain. Stakeholders are rapidly innovating to meet not just regulatory compliance but also patient expectations for safer medications. The growing complexity of global drug distribution, especially with the rise of biologics, specialty therapies, and direct-to-patient models, continues to fuel demand for packaging formats that guarantee product authenticity at every point of transit. Moreover, sustainability goals are reshaping the way manufacturers approach materials and designs, placing a dual focus on security and environmental responsibility.

As pharmaceutical needs evolve, tamper-proof packaging solutions are becoming essential to safeguard products from contamination, counterfeiting, and damage during transport. Rising regulatory pressures and consumer demand for verified safe medicines are propelling innovations in materials and technologies. Shifts in trade policies have further impacted the market's cost structure, pushing manufacturers to rethink their sourcing models. With tariffs and cross-border restrictions adding financial and logistical strain, companies are moving toward nearshoring and reshoring strategies to stabilize supply chains. This trend enables faster production timelines, enhanced quality control, and quicker compliance with regional safety regulations. To stay ahead of tightening mandates, packaging suppliers are investing heavily in serialization technologies, advanced tamper-evident features, and blockchain-based tracking systems that boost product authenticity and simplify regulatory audits, ultimately strengthening trust across the distribution chain.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $13.4 Billion |
| Forecast Value | $24.6 Billion |
| CAGR | 6.4% |
The plastic material segment led the pharmaceutical tamper proof packaging market in 2024, generating USD 5.9 billion in revenue. Plastics dominate the space due to their adaptability across bottles, blister packs, closures, and ampoules for solid and liquid medications. Their lightweight nature, cost-efficiency, and ease of mass production continue to make them the preferred choice. At the same time, manufacturers are increasingly adopting biodegradable and recyclable variants such as PLA, PET, and HDPE to align with global eco-friendly initiatives and evolving regulatory frameworks.
Pharmaceutical manufacturers accounted for USD 9.7 billion in 2024, driven by the rising integration of tamper-proof packaging in compliance with regional serialization and authentication regulations. Companies are reinforcing supply chains with smart packaging featuring tracking systems and breach detection, especially for biologics and chronic care therapies that demand specialized handling formats.
The U.S. pharmaceutical tamper proof packaging market reached USD 4 billion in 2024. Stricter enforcement by regulatory bodies and the growing emphasis on patient safety are fueling the widespread deployment of shrink bands, RFID seals, and forensic labeling. The boom in e-pharmacies and remote patient care models is pushing for heightened packaging integrity, especially for temperature-sensitive and high-risk medications.
Major players in the global pharmaceutical tamper proof packaging market include NanoMatriX Technologies Limited, Applied DNA Sciences, Inc., Lonza, Tamperguard, 3M, SATO Holdings Corporation, WestRock Company, SICPA HOLDING SA, CCL Industries Inc., SML Group, ACG, Authentix, Alien Technology Corp., Avery Dennison Corporation, CordenPharma, AlpVision SA, Systech, and Essentra plc. Companies are investing in digital serialization, blockchain authentication, recyclable packaging, and automation in sealing technologies to stay competitive. Collaborations with pharmaceutical brands for tailored tamper-resistant solutions and increasing R&D investments in sustainable materials are shaping the future of the market.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Trump administration tariffs
- 3.2.1 Impact on trade
- 3.2.1.1 Trade volume disruptions
- 3.2.1.2 Retaliatory measures
- 3.2.2 Impact on the industry
- 3.2.2.1 Supply-side impact (raw materials)
- 3.2.2.1.1 price volatility in key materials
- 3.2.2.1.2 Supply chain restructuring
- 3.2.2.1.3 Production cost implications
- 3.2.2.2 Demand-side impact (selling price)
- 3.2.2.2.1 Price transmission to end markets
- 3.2.2.2.2 Market share dynamics
- 3.2.2.2.3 Consumer response patterns
- 3.2.2.1 Supply-side impact (raw materials)
- 3.2.3 Key companies impacted
- 3.2.4 Strategic industry responses
- 3.2.4.1 Supply chain reconfiguration
- 3.2.4.2 Pricing and product strategies
- 3.2.4.3 Policy engagement
- 3.2.5 Outlook and future considerations
- 3.2.1 Impact on trade
- 3.3 Industry impact forces
- 3.3.1 Growth drivers
- 3.3.1.1 Rising global medicine demand
- 3.3.1.2 Advancements in packaging technology
- 3.3.1.3 E-commerce expansion in pharma
- 3.3.1.4 Increased investment in R&D
- 3.3.1.5 Expansion of cold chain logistics
- 3.3.2 Industry pitfalls and challenges
- 3.3.2.1 High cost of implementation
- 3.3.2.2 Environmental concerns
- 3.3.1 Growth drivers
- 3.4 Growth potential analysis
- 3.5 Regulatory landscape
- 3.6 Technology landscape
- 3.7 Future market trends
- 3.8 Gap analysis
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Packaging Type, 2021 - 2034 (USD Million & Kilo Tons)
- 5.1 Key trends
- 5.2 Blister packaging
- 5.3 Strip packaging
- 5.4 Bottles & jars
- 5.5 Vials & ampoules
- 5.6 Sachets & pouches
- 5.7 Cartons
- 5.8 Syringes
- 5.9 Others
Chapter 6 Market Estimates and Forecast, By Tamper Evident Technology, 2021 - 2034 (USD Million & Kilo Tons)
- 6.1 Key trends
- 6.2 Induction sealing
- 6.3 Breakable caps/closures
- 6.4 Film wrappers
- 6.5 Blister foils
- 6.6 Tamper-evident labels & seals
- 6.7 Electronic track-and-trace systems
- 6.8 Others
Chapter 7 Market Estimates and Forecast, By Material Type, 2021 - 2034 (USD Million & Kilo Tons)
- 7.1 Key trends
- 7.2 Plastic
- 7.2.1 PET
- 7.2.2 HDPE
- 7.2.3 PVC
- 7.2.4 Others
- 7.3 Glass
- 7.4 Paper & paperboard
- 7.5 Aluminum foil
- 7.6 Others
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 (USD Million & Kilo Tons)
- 8.1 Key trends
- 8.2 Pharmaceutical manufacturers
- 8.3 Contract packaging organizations (CPOs)
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 (USD Million & Kilo Tons)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 3M
- 10.2 ACG
- 10.3 Alien Technology
- 10.4 AlpVision
- 10.5 Applied DNA Sciences
- 10.6 Authentix
- 10.7 Avery Dennison
- 10.8 CCL Industries
- 10.9 CordenPharma
- 10.10 Essentra
- 10.11 Lonza
- 10.12 NanoMatrix Technologies
- 10.13 SATO Holdings
- 10.14 SICPA Holding
- 10.15 SML Group
- 10.16 Systech
- 10.17 Tamperguard
- 10.18 WestRock











