 |
市場調查報告書
商品編碼
1891246
疫苗市場:產業趨勢及全球預測(至 2035 年)-依疫苗原料藥類型、目標病患群體、疫苗類型、給藥途徑及主要地區劃分Vaccines Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Type of Vaccine API, Targeted Patient Population, Type of Vaccines, Route of Administration and Key Geographical Region |
||||||
疫苗市場概覽
預計到 2030 年,疫苗市場規模將從目前的 480 億美元增長至 940 億美元,預測期內(至 2030 年)複合年增長率 (CAGR) 為 11.9%。
疫苗市場
市場規模與機會分析依下列參數細分:
疫苗原料藥類型
- 結合疫苗
- 滅活疫苗和次單位疫苗
- 減毒活疫苗
- 重組疫苗
- 類毒素疫苗
- 其他
目標族群
- 兒童
- 成人
疫苗類型
- 肺炎鏈球菌結合疫苗
- 人類乳突病毒疫苗
- 流感疫苗
- 輪狀病毒疫苗
- 水痘疫苗
- 百白破-肝炎疫苗B-Hib聯合疫苗
- 其他
給藥途徑
- 肌肉注射
- 皮下注射
- 口服
- 靜脈注射
- 其他
主要地區
- 北美
- 歐洲
- 世界其他地區
疫苗市場 - 成長與趨勢
隨著傳染病威脅的日益加劇,全球對疫苗的需求也不斷增長。疫苗是一種生物製品,由減毒或滅活的微生物及其表面蛋白質或毒素製成。這些疫苗能夠提供針對特定疾病的主動免疫,使免疫系統在未來再次遇到相同病原體時產生強烈的免疫反應。如今,接種疫苗已成為降低傳染病風險和全球死亡率的關鍵手段。全球傳染病疫情凸顯了改善疫苗以降低疾病風險的必要性。世界衛生組織(世衛組織)近期發布了一份關於疾病爆發的報告,指出禽流感A和猴痘的感染人數不斷增加。
此外,歐洲疾病預防控制中心(ECDC)報告稱,全球約有450萬例登革熱確診病例,造成約4000人死亡。世衛組織也報告稱,全球每年約有64種新的傳染病影響著人們。每年約有200萬人因輪狀病毒感染而住院。面對這些情況,許多產業領袖、政府機構和公共衛生組織已啟動各種免疫接種計劃,並致力於研發先進疫苗。近期,Biovac和Sanfoy宣布合作在非洲生產滅活小兒麻痺疫苗。該合作旨在提高小兒麻痺疫苗的產量,以滿足40多個非洲國家日益增長的需求。
此外,法國總統馬克宏與多位非洲領導人共同決定啟動一項11億美元的計劃,旨在加速非洲國家的疫苗生產。目前,各利益相關者正在啟動多個研究項目,以促進醫學創新並降低傳染病傳播的風險。在疫苗需求持續成長和合作不斷擴大的推動下,預計疫苗市場將在預測期內穩步擴張。
疫苗市場-關鍵洞察
本報告深入分析了疫苗市場的現狀,並指出了該行業的潛在成長機會。主要發現包括:
- 目前,由國內外參與者開發的200多種預防性疫苗正在進行臨床開發評估。
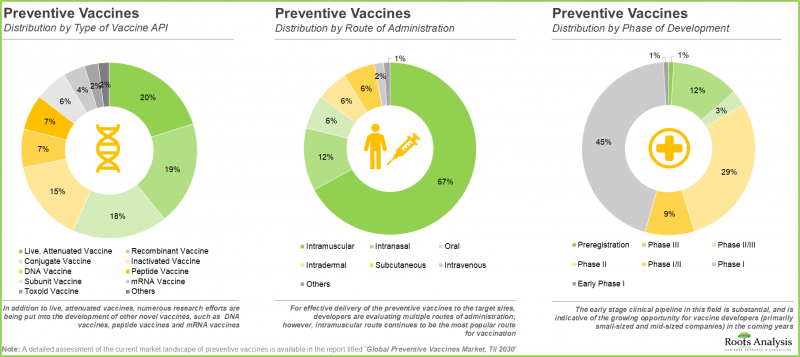
- 目前,多種疫苗活性成分 (API) 正被研究用於不同的給藥途徑,其中大多數仍處於早期研發階段。
- 為了建立競爭優勢,疫苗研發人員正投入大量精力,以確保其候選疫苗的臨床和商業可行性。
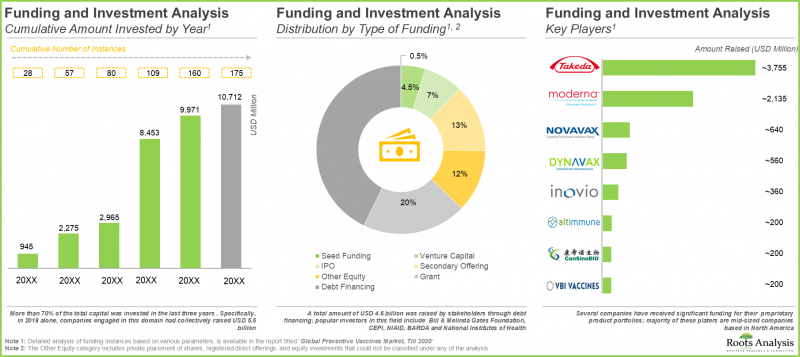
- 鑑於該領域的潛力,自 2015 年以來,私人和公共投資者已向 175 個疫苗研發項目投資超過 100 億美元。
- 近年來,已註冊超過 1400 項評估各類預防性疫苗的臨床試驗,顯示該領域發展迅速。
- 全球約有 70 家公司聲稱除了生產服務外,還提供合約開發、灌裝和包裝以及法規支援。
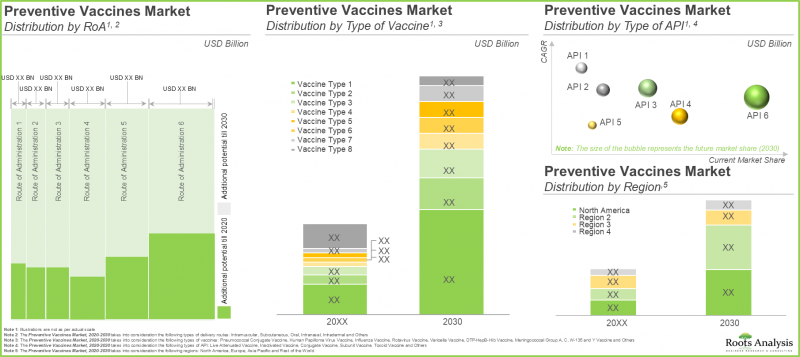
- 預計到2030年,疫苗市場將以11.9%的複合年增長率成長,潛在機會涵蓋各種給藥途徑、疫苗類型和主要地區。
疫苗市場 - 主要細分市場
預計減毒活疫苗細分市場將佔大部分市場佔有率
依技術類型劃分,全球疫苗市場可細分為結合疫苗、滅活疫苗和次單位疫苗、減毒活疫苗、重組疫苗、類毒素疫苗和其他疫苗。受對抗各種傳染病的強效疫苗需求不斷增長的推動,減毒活疫苗佔了最大的市場佔有率,預計今年將佔疫苗市場整體佔有率的28%。由於新型疫苗(例如DNA疫苗)的研究不斷深入,其他類型疫苗在預測期內的複合年增長率預計將達到16.8%。
兒科疫苗市場佔最大佔有率
根據目標患者的人口統計數據,全球疫苗市場分為兒科疫苗和成人疫苗。根據我們的市場調查,兒科疫苗市場今年將佔疫苗市場整體佔有率的最大部分(56%)。兒科疫苗市場的成長主要歸功於對嬰幼兒疫苗接種的日益重視以及政府加強推廣兒童疫苗接種。長期來看,受地方性流行病和全球性流行病病例增加的推動,預計成人疫苗市場在預測期內將以12.1%的複合年增長率高速成長。
目前,肌肉注射途徑佔主導地位。
依給藥途徑,疫苗市場可分為肌肉注射、皮下注射、口服、靜脈注射及其他途徑。據我們預測,今年肌肉注射途徑將佔疫苗市場的大部分佔有率(52%)。這一增長主要得益於人們對腸外疫苗的偏好日益增長,而腸外疫苗可以繞過胃腸道。肌肉注射具有給藥方便、副作用少等優點。長期來看,靜脈注射疫苗市場預計在預測期內將以14.6%的複合年增長率(CAGR)成長。
預計麻疹、腮腺炎、德國麻疹(MMR)疫苗在預測期內將以12.3%的複合年增長率(CAGR)增長。
依疫苗類型劃分,市場可分為肺炎鏈球菌結合疫苗、人類乳突病毒疫苗、輪狀病毒疫苗、流感疫苗、MMR疫苗、破傷風-白喉加強疫苗、水痘疫苗、DTaP-Hib-IPV疫苗、DTaP-HepB-Hib-IPV疫苗及其他疫苗。受傳染病發生率上升的推動,預計其他疫苗類別今年將佔最大的疫苗市場佔有率(32.4%)。此外,肺炎鏈球菌結合疫苗目前佔第二大市場佔有率(25.8%)。輝瑞的沛兒(Prevnar)系列疫苗和默克公司在肺炎鏈球菌疫苗市場佔領先地位。默克近期獲得了美國FDA對其21價肺炎球菌疫苗CAPVAXIVE的批准。長期來看,麻疹、腮腺炎、德國麻疹(MMR)疫苗預計在預測期內將以12.3%的複合年增長率(CAGR)高速成長。 北美地區今年將佔全球疫苗市場最大的收入佔有率。 依主要地區劃分,全球市場分為北美、歐洲、亞太地區和其他地區。根據我們的預測,北美地區今年將佔最大的市場佔有率(44%)。該地區疫苗市場成長的主要驅動因素包括:對疫苗接種的日益重視、醫療保健行業的快速發展以及美國疫苗監測力度的加強。然而,亞太地區的疫苗需求預計也將成長,在預測期內將以14.7%的複合年增長率高速成長。 2023年2月,印度政府與全球疫苗免疫聯盟(Gavi)簽署了一項為期三年的新合作協議,為印度數百萬名兒童提供合適的疫苗。根據協議,Gavi將出資2.5億美元,用於識別和接種未接種疫苗的兒童,加強衛生系統建設,並支持將人類乳頭瘤病毒疫苗(HPV)和傷寒結合疫苗(TCV)納入印度的國家免疫規劃。
主要公司市佔率
該行業的主要公司包括Bio Farma、Emergent BioSolutions、GC Pharma、葛蘭素史克、強生、默克、諾瓦瓦克斯、莫德納、輝瑞、賽諾菲巴斯德和Valneva。葛蘭素史克在本財年佔了疫苗市場21%的佔有率。該公司之所以能佔如此高的市場佔有率,很可能是因為其疫苗產品組合豐富,並且在疫苗領域加速了研發進程。
疫苗市場代表性公司
- Bio Farma
- Emergent BioSolutions
- GC Pharma
- 葛蘭素史克
- 強生
- 默克
- 諾瓦瓦克斯
- 摩德納
- 輝瑞
- 賽諾菲巴斯德
- 瓦爾內瓦
疫苗市場概覽
- 市場規模及機會分析:本報告對疫苗市場進行了詳細分析,重點關注以下關鍵市場細分:[A] 疫苗活性成分類型,[B] 目標人群,[C] 疫苗類型,[D] 給藥途徑,以及 [E] 主要地區。
- 市場概況:基於多項參數,對目前處於不同研發階段的200多種預防性疫苗的現狀進行詳細評估,這些參數包括:[A] 研發公司類型,[B] 主要候選疫苗的研發階段,[C] 給藥途徑,[D] 疫苗活性成分類型,[E] 劑型/劑量,[F] 目標疾病,[D] 目標群體。
- 公司競爭分析:對總部位於北美、歐洲和亞太地區的預防性疫苗研發公司進行全面的競爭分析,考察[A] 供應能力,[B] 研發管線實力等因素。
- 公司簡介:詳細介紹總部位於北美、歐洲和亞太地區的領先預防性疫苗研發和生產商,重點關注[A] 成立年份,[B] 總部所在地,[C] 產品組合,[D] 近期發展,以及 [E] 未來展望。
- 臨床試驗分析:針對已完成、正在進行和計劃中的預防性疫苗臨床試驗,包括:[A] 研究入組年份,[B] 研發階段,[C] 受試者招募狀態,[D] 研究設計,[E] 研究目標領域,[F] 預防性疫苗類型,[G] 目標疾病適應症,[E] 研究目標領域,[F] 預防性疫苗類型,[G] 目標疾病適應症,[E] 合作,I/? [K] 地理分佈。
- 複雜疾病疫苗研發進展:概述針對複雜疾病(例如 [A] COVID-19,[B] 伊波拉病毒疾病,[C] HIV/AIDS,[D] 瘧疾,以及 [E] 寨卡病毒感染)的正在進行的疫苗研發進展。內容包括疾病資訊、全球負擔、目前治療狀況以及預防性疫苗研究進展。
目錄
第一章:前言
第二章:摘要整理
第三章:導論
- 章節概述
- 預防性疫苗
第四章:市場概況
- 章節概述
- 預防性疫苗:市場概況
第五章:競爭分析
- 章節概述
- 研究方法
- 假設和關鍵參數
- 競爭分析:預防性疫苗研發企業
第六章:公司公司簡介
- 章節概述
- Bio Farma
- Emergent BioSolutions
- GC Pharma
- 葛蘭素史克
- 強生
- 默克
- 諾瓦瓦克斯
- 輝瑞
- 賽諾菲巴斯德
- 瓦爾內瓦
第七章:臨床試驗分析
- 章節概述
- 研究範圍與方法
- 預防性疫苗:臨床試驗分析
第八章:針對複雜疾病的持續疫苗研發計畫
- 章節概述
- 冠狀病毒疾病(COVID-19)
- 伊波拉病毒病 (EVD)
- 愛滋病毒/愛滋病
- 瘧疾
- 寨卡病毒病
第九章 資金與投資分析
- 章節概述
- 資金類型
- 預防性疫苗:資金與投資分析
第十章:市場規模與機會分析
- 章節概述
- 預測研究方法與關鍵假設
- 2030 年預防性疫苗市場總量
第十一章:案例研究:疫苗合約生產
- 章節概述
- 疫苗合約生產
- 疫苗合約生產:市場景觀
第十二章:結論
第十三章:高階主管洞察
第十四章:附錄一:表格資料
第十五章:附錄二:公司與組織清單
Vaccines Market: Overview
As per Roots Analysis, the vaccines market is estimated to grow from USD 48 billion in the current year to USD 94 billion by 2030, at a CAGR of 11.9% during the forecast period, till 2030.
Vaccines Market
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Vaccine API
- Conjugate Vaccines
- Inactivated and Subunit Vaccines
- Live Attenuated Vaccines
- Recombinant Vaccines
- Toxoid Vaccines
- Others
Targeted Patient Population
- Pediatric
- Adult
Type of Vaccines
- Pneumococcal Conjugate Vaccine
- Human Papillomavirus Vaccine
- Influenza Vaccine
- Rotavirus Vaccine
- Varicella Vaccine
- DTP-HepB-Hib Vaccine
- Others
Route of Administration
- Intramuscular
- Subcutaneous
- Oral Administration
- Intravenous
- Others
Key Geographical Region
- North America
- Europe
- Rest of the World
Vaccines Market: Growth and Trends
With the increasing threat of infectious diseases, there has been a rise in the need for vaccines worldwide. A vaccine is a type of biological formulation created using weakened or inactive microbes, along with their surface proteins and toxins. These vaccines offer active acquired immunity against specific diseases and allow the immune system to produce a robust response if a person encounters the same pathogen in the future. Vaccination is now a critical means of lowering the risk of infectious diseases and decreasing global mortality rates. The global emergence of infectious diseases has highlighted the need for improved vaccines to mitigate disease risks. Recently, the World Health Organization published a report on disease outbreaks, revealing the rising number of people affected by Avian influenza A and Mpox.
Moreover, the European Centre for Disease Prevention and Control reported that approximately 4.5 million dengue cases had been documented worldwide, with about 4,000 fatalities confirmed. In addition, the World Health Organization announced approximately 64 new infectious diseases impacting individuals worldwide. Further, approximately 2 million individuals affected by rotavirus are admitted to the hospital annually. In this context, numerous industry leaders, governmental bodies, and public health organizations have launched diverse immunization initiatives and concentrated on creating advanced vaccines. In was also recently reported that Biovac and Sanfoi established a collaboration for the manufacturing of inactivated polio vaccines in Africa. The aim of the collaboration is to increase the production of polio vaccines to satisfy the rising need for these vaccines in more than 40 African nations.
Additionally, French President Emmanuel Macron opted to collaborate with several African leaders in organizing a USD 1.1 billion initiative aimed at speeding up vaccine production in African nations. At present, various research projects have been launched by stakeholders to promote innovation in the healthcare sector and reduce the risk of transmitting infectious diseases. Due to the continuous need for vaccines and rising collaborations, the vaccine market is expected to expand at a consistent pace during the forecast period.
Vaccines Market: Key Insights
The report delves into the current state of the vaccines market and identifies potential growth opportunities within industry. Some key findings from the report include:
- More than 200 preventive vaccines, developed by both industry and non-industry players, are being evaluated in clinical stages of development.

- A variety of vaccine APIs, designed for administration via multiple routes of delivery, are presently being investigated; most such candidates are in the early stages of development.
- In order to achieve a competitive edge, vaccine developers are putting in significant efforts to ensure that their candidates are clinically and commercially competent.
- Foreseeing a lucrative future in this domain, several private and public investors have invested over USD 10 billion in vaccine development initiatives, across 175 instances, in the time period since 2015.

- Over the last few years, 1,400+ clinical trials evaluating various types of preventive vaccines have been registered, indicating the rapid pace of development in this field.
- Around 70 companies, situated in different regions across the globe, claim to provide contract development, fill / finish and regulatory support, in addition to manufacturing services.

- The market is anticipated to grow at a CAGR of 11.9%, till 2030, and the projected opportunity is likely to be distributed across various routes of administration, vaccine types and key geographical regions.
Vaccines Market: Key Segments
Live Attenuated Vaccines Segment is Likely to Capture the Majority of the Market Share
In terms of the type of technology, the global market for vaccines is segmented into conjugate vaccines, inactivated and subunit vaccines, live attenuated vaccines, recombinant vaccines, toxoid vaccines, and others. Fueled by the rising need for potent vaccines to combat various infectious diseases, live attenuated vaccines are projected to occupy the largest segment, securing 28% of the total vaccines market share in the current year. Other types of vaccines will grow at a higher CAGR of 16.8% during the forecast period owing to the growing research on novel vaccines such as DNA vaccines.
Pediatric Segment Holds the Highest Vaccines Market Share
In terms of target patient population, the global market has been distributed into pediatrics and adults. According to our market study, the pediatric segment accounts for the largest share (56%) of the vaccines market in the current year. The expansion of the pediatric market segment is due to the rising emphasis on immunizing infants against illnesses and the increasing government efforts to promote vaccination among children. In the long run, the adult segment will grow at a higher CAGR of 12.1% during the forecast period, driven by the increasing cases of endemic and pandemics.
The Current Market Share is Captured by Intramuscular Segment
In terms of route of administration, the vaccines market is segmented into intramuscular, subcutaneous, oral, intravenous and others. According to our projection, the intramuscular segment capture majority (52%) of the overall vaccine market in the current year. The increase can be attributed to the rising preference for parenteral vaccines, as they can avoid the gastrointestinal tract. The intramuscular route facilitates easy administration and produces fewer side effects. In the long term, the intravenous segment is expected to grow at a higher CAGR of 14.6% throughout the forecast period.
MMR Vaccines will Grow at a Higher CAGR of 12.3% during the Forecast Period
In terms of the type of vaccines, the market is segmented into Pneumococcal Conjugate Vaccine, Human Papillomavirus Vaccine, Rotavirus Vaccine, Influenza Vaccine, MMR Vaccine, Tetanus and Diphtheria Booster Vaccine, Varicella Vaccine, DTaP-Hib-IPV Vaccine, DTaP-HepB-Hib-IPV Vaccine and other. Driven by the growing incidences of infectious diseases, the others segment accounts to hold the largest (32.4%) vaccines market share in the current year. Additionally, the pneumococcal conjugate vaccine accounts to hold the second highest share 25.8% of the market currently. Pfizer with its Prevnar series and Merck are the market leaders in pneumococcal vaccine market. Merck received US FDA approval for its 21-valent pneumococcal vaccine, CAPVAXIVE recently. In the long run, MMR vaccines will grow at a higher CAGR of 12.3% during the forecast period.
North America Accounts for the Highest Revenues in the Global Vaccines Market in the Current Year
In terms of key geographies, the global market has been distributed across North America, Europe, Asia-Pacific and Rest of the World. According to our projections, North America accounts for the largest share (44%) of the market in the current year. The primary factors driving the growth of the vaccine market in this area are the heightened focus on vaccination implementation, the fast-expanding healthcare industry, and a rise in vaccine research in the US. However, the demand for vaccines in Asia-Pacific is estimated to witness growth and is likely to grow at a substantial CAGR of 14.7% during the forecast period. In February 2023, the Government of India forged a new three-year partnership with Gavi (Leading Vaccine Alliance) with the aim of immunizing millions of children in India with appropriate vaccines. Under this agreement, Gavi has allocated USD 250 million for the identification and vaccination of children who haven't received any vaccine strengthening healthcare systems and supporting the introduction of HPV (human papillomavirus vaccine) along with TCV (typhoid conjugate vaccine) into India's national routine immunization schedule.
Market Share by Key Players
The key players active in this industry are Bio Farma, Emergent BioSolutions, GC Pharma, GlaxoSmithKline, Janssen, Merck, Novavax, Moderna, Pfizer, Sanofi Pasteur and Valneva. Currently, GlaxoSmithKline accounts to hold 21% of the overall vaccines market share in the current year. The highest share can be attributed to its extensive range of vaccines and GlaxoSmithKline's acceleration of research and development efforts in the vaccine sector.
Example Players in Vaccines Market
- Bio Farma
- Emergent BioSolutions
- GC Pharma
- GlaxoSmithKline
- Janssen
- Merck
- Novavax
- Moderna
- Pfizer
- Sanofi Pasteur
- Valneva
Vaccines Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the vaccines market, focusing on key market segments, including [A] type of vaccine API, [B] targeted patient population, [C] type of vaccines, [D] route of administration, and [E] key geographical regions.
- Market Landscape: A detailed assessment of the current market landscape of over 200 preventive vaccines that are currently being evaluated in different stages of development, based on a number of parameters, such as [A] type of developer, [B] phase of development of lead candidates, [C] route of administration, [D] type of vaccine API, [E] dosage form, dosage, [F] target disease indication and [G] target patient population.
- Company Competitiveness Analysis: A comprehensive competitive analysis of of preventive vaccine developers based in North America, Europe and Asia-Pacific, examining factors, such as [A] supplier strength and [B] pipeline strength.
- Company Profiles: In-depth profiles of the key preventive vaccine developers based in North America, Europe and Asia-Pacific that are engaged in manufacturing focusing on [A] year of establishment, [B] location of headquarters, [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Clinical Trial Analysis: A detailed analysis of various completed, ongoing and planned clinical studies of preventive vaccines based on various relevant parameters, including [A] trial registration year, [B] phase of development, [C] trial recruitment status, [D] study design, [E] trial focus area, [F] type of preventive vaccine, [G] target disease indication(s), [H] type of sponsor / collaborator, [I] leading industry sponsors / collaborators, [J] enrolled patients population and [K] regional distribution.
- Ongoing Vaccine Development Initiatives for Complex Conditions: An overview of the ongoing vaccine development initiatives for complex conditions, such as [A] COVID-19, [B] Ebola virus disease, [C] HIV / AIDS, [D] malaria and [E] zika virus infection, including information on disease, its global burden, current treatment landscape and preventive vaccine research landscape.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What is the current global capacity of developers?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Preventive Vaccines
- 3.2.1. Classification of Vaccines
- 3.2.1.1. Live, Attenuated Vaccines
- 3.2.1.2. Inactivated Vaccines
- 3.2.1.3. Subunit Vaccines
- 3.2.1.4. Toxoid Vaccines
- 3.2.1.5. DNA Vaccines
- 3.2.2. Key Components of a Vaccine Formulation
- 3.2.3. Production of Vaccines using Different Expression Systems
- 3.2.3.1. Embryonated Chicken Eggs and Primary Chicken Embryonic Fibroblasts (CEFs)
- 3.2.3.2. Mammalian Expression Systems
- 3.2.3.3. Avian Expression Systems
- 3.2.3.4. Plant Expression Systems
- 3.2.3.5. Bacterial Expression Systems
- 3.2.3.6. Yeast Expression Systems
- 3.2.3.7. Insect Expression System
- 3.2.4. Routes of Vaccine Administration
- 3.2.4.1. Intramuscular Route
- 3.2.4.2. Subcutaneous Route
- 3.2.4.3. Oral Route
- 3.2.4.4. Intranasal Route
- 3.2.4.5. Intradermal Route
- 3.2.4.6. Inhalation
- 3.2.5. Clinical Development and Approval of Vaccines
- 3.2.6. Future Perspectives
- 3.2.1. Classification of Vaccines
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Preventive Vaccines: Overall Market Landscape
- 4.2.1. Marketed Vaccines Landscape
- 4.2.2. Clinical-Stage Vaccines Landscape
- 4.2.2.1. Analysis by Type of Developer
- 4.2.2.2. Analysis by Phase of Development
- 4.2.2.3. Analysis by Route of Administration
- 4.2.2.4. Analysis by Type of Vaccine API
- 4.2.2.5. Analysis by Dosage Form
- 4.2.2.6. Analysis by Dosage
- 4.2.2.7. Analysis by Target Disease Indication
- 4.2.2.8. Analysis by Target Patient Population
- 4.2.2.9. Key Industry Players: Analysis by Number of Vaccines in Clinical Development
- 4.2.2.10. Key Non-Industry Players: Analysis by Number of Vaccines in Clinical Development
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Methodology
- 5.3. Assumptions and Key Parameters
- 5.4. Competitiveness Analysis: Preventive Vaccine Developers
- 5.4.1. Preventive Vaccine Developers based in North America
- 5.4.2. Preventive Vaccine Developers based in Europe
- 5.4.3. Preventive Vaccine Developers based in Asia Pacific
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Bio Farma
- 6.2.1. Company Overview
- 6.2.2. Preventive Vaccines Portfolio
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Emergent BioSolutions
- 6.3.1. Company Overview
- 6.3.2. Preventive Vaccines Portfolio
- 6.3.3. Recent Developments and Future Outlook
- 6.4. GC Pharma
- 6.4.1. Company Overview
- 6.4.2. Preventive Vaccines Portfolio
- 6.4.3. Recent Developments and Future Outlook
- 6.5. GlaxoSmithKline
- 6.5.1. Company Overview
- 6.5.2. Preventive Vaccines Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. Janssen
- 6.6.1. Company Overview
- 6.6.2. Preventive Vaccines Portfolio
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Merck
- 6.7.1. Company Overview
- 6.7.2. Preventive Vaccines Portfolio
- 6.7.3. Recent Developments and Future Outlook
- 6.8. Novavax
- 6.8.1. Company Overview
- 6.8.2. Preventive Vaccines Portfolio
- 6.8.3. Recent Developments and Future Outlook
- 6.9. Pfizer
- 6.9.1. Company Overview
- 6.9.2. Preventive Vaccines Portfolio
- 6.9.3. Recent Developments and Future Outlook
- 6.10. Sanofi Pasteur
- 6.10.1. Company Overview
- 6.10.2. Preventive Vaccines Portfolio
- 6.10.3. Recent Developments and Future Outlook
- 6.11. Valneva
- 6.11.1. Company Overview
- 6.11.2. Preventive Vaccines Portfolio
- 6.11.3. Recent Developments and Future Outlook
7. CLINICAL TRIAL ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Preventive Vaccines: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Enrolled Patient Population and Trial Registration Year
- 7.3.3. Analysis by Trial Phase
- 7.3.4. Analysis by Trial Recruitment Status
- 7.3.5. Analysis by Study Design
- 7.3.6. Analysis by Trial Focus Area
- 7.3.7. Analysis by Type of Preventive Vaccine (based on Pathogen)
- 7.3.8. Analysis by Target Disease Indication
- 7.3.9. Analysis by Type of Sponsor / Collaborator
- 7.3.10. Leading Industry Players: Analysis by Number of Registered Trials
- 7.3.11. Geographical Analysis by Number of Registered Trials
- 7.3.12. Geographical Analysis by Enrolled Patient Population
- 7.3.13. Geographical Analysis by Trial Recruitment Status
8. ONGOING VACCINE DEVELOPMENT INITIATIVES FOR COMPLEX CONDITIONS
- 8.1. Chapter Overview
- 8.2. Coronavirus Disease (COVID-19)
- 8.2.1. Disease Overview
- 8.2.2. Global Burden of COVID-19
- 8.2.3. Current Treatment Landscape
- 8.2.4. Preventive Vaccines for COVID-19
- 8.2.4.1. Historical Background of COVID-19 Vaccine Research
- 8.2.4.2. COVID-19 and Affiliated Research Landscape
- 8.2.5. Funding Instances
- 8.2.6. Recent Developments
- 8.3. Ebola Virus Disease (EVD)
- 8.3.1. Disease Overview
- 8.3.2. Global Burden of EVD
- 8.3.3. Current Treatment Landscape
- 8.3.4. Preventive Vaccines for EVD
- 8.3.4.1. Historical Background of Ebola Virus Vaccine Research
- 8.3.4.2. Anti-Ebola Virus Vaccines and Affiliated Research Landscape
- 8.3.5. Funding Instances
- 8.3.6. Recent Developments
- 8.4. HIV/AIDS
- 8.4.1. Disease Overview
- 8.4.2. Global Burden of HIV/AIDS
- 8.4.3. Current Treatment Landscape
- 8.4.4. Preventive Vaccines for HIV/AIDS
- 8.4.4.1. Historical Background of HIV/AIDS Vaccine Research
- 8.4.4.2. Anti-HIV Vaccines and Affiliated Research Landscape
- 8.4.5. Funding Instances
- 8.4.6. Recent Developments
- 8.5. Malaria
- 8.5.1. Disease Overview
- 8.5.2. Global Burden of Malaria
- 8.5.3. Current Treatment Landscape
- 8.5.4. Preventive Vaccines for Malaria
- 8.5.4.1. Historical Background of Malaria Vaccine Research
- 8.5.4.2. Anti-Malaria Vaccines and Affiliated Research Landscape
- 8.5.5. Funding Instances
- 8.5.6. Recent Developments
- 8.6. Zika Virus Infection
- 8.6.1. Disease Overview
- 8.6.2. Global Burden of Zika Virus Infection
- 8.6.3. Current Treatment Landscape
- 8.6.4. Preventive Vaccines for Zika Virus Infection
- 8.6.4.1. Historical Background of Zika Virus Vaccine Research
- 8.6.4.2. Anti-Zika Virus Vaccines and Affiliated Research Landscape
- 8.6.5. Funding Instances
- 8.6.6. Recent Developments
9. FUNDING AND INVESTMENT ANALYSIS
- 9.1. Chapter Overview
- 9.2. Types of Funding
- 9.3. Preventive Vaccines: Funding and Investment Analysis
- 9.3.1. Analysis by Number of Funding Instances
- 9.3.2. Analysis by Amount Invested
- 9.3.3. Analysis by Type of Funding
- 9.3.4. Analysis by Amount Invested across Different Types of Vaccine API
- 9.3.5. Analysis by Focus Area
- 9.3.6. Analysis by Amount Invested by Different Type of Investors
- 9.3.7. Most Active Players: Analysis by Number of Funding Instances
- 9.3.8. Most Active Investors: Analysis by Number of Funding Instances
- 9.3.9. Analysis by Geography
- 9.3.9.1. Continent-wise Analysis
- 9.3.9.2. Country-wise Analysis
10. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 10.1. Chapter Overview
- 10.2. Forecast Methodology and Key Assumptions
- 10.3. Overall Preventive Vaccines Market, till 2030
- 10.3.1. Preventive Vaccines Market, till 2030: Distribution by Route of Administration
- 10.3.2. Preventive Vaccines Market, till 2030: Distribution by Type of Vaccine
- 10.3.3. Preventive Vaccines Market, till 2030: Distribution by Type of Vaccine API
- 10.3.4. Preventive Vaccines Market, till 2030: Distribution by Target Patient Population
- 10.3.5. Preventive Vaccines Market, till 2030: Distribution by Key Geographical Regions
- 10.3.5.1. Preventive Vaccines Market in North America, till 2030
- 10.3.5.1.1. Preventive Vaccines Market in the US, till 2030
- 10.3.5.1.2. Preventive Vaccines Market in Mexico, till 2030
- 10.3.5.1.3. Preventive Vaccines Market in Canada, till 2030
- 10.3.5.2. Preventive Vaccines Market in Europe, till 2030
- 10.3.5.2.1. Preventive Vaccines Market in Spain, till 2030
- 10.3.5.2.2. Preventive Vaccines Market in the UK, till 2030
- 10.3.5.2.3. Preventive Vaccines Market in Italy, till 2030
- 10.3.5.2.4. Preventive Vaccines Market in France, till 2030
- 10.3.5.2.5. Preventive Vaccines Market in Germany, till 2030
- 10.3.5.2.6. Preventive Vaccines Market in Rest of Europe, till 2030
- 10.3.5.3. Preventive Vaccines Market in Asia Pacific, till 2030
- 10.3.5.3.1. Preventive Vaccines Market in India, till 2030
- 10.3.5.3.2. Preventive Vaccines Market in China, till 2030
- 10.3.5.3.3. Preventive Vaccines Market in Australia, till 2030
- 10.3.5.3.4. Preventive Vaccines Market in Rest of Asia Pacific, till 2030
- 10.3.5.4. Preventive Vaccines Market in Rest of the World, till 2030
- 10.3.5.1. Preventive Vaccines Market in North America, till 2030
11. CASE-IN-POINT: CONTRACT MANUFACTURING OF VACCINES
- 11.1. Chapter Overview
- 11.2. Vaccine Contract Manufacturing
- 11.2.1. Addressing an Unmet Need
- 11.2.2. Commonly Outsourced Operations
- 11.2.3. Selecting a CMO Partner
- 11.2.4. Advantages of Outsourcing Manufacturing Services
- 11.2.5. Associated Risks and Challenges
- 11.3. Vaccine Contract Manufacturing: Overall Market Landscape
- 11.3.1. Analysis by Year of Establishment
- 11.3.2. Analysis by Company Size
- 11.3.3. Analysis by Scale of Operation
- 11.3.4. Analysis by Location of Headquarters
- 11.3.5. Analysis by Location of Manufacturing Facilities
- 11.3.6. Analysis by Type of Service(s) Offered
- 11.3.7. Analysis by Expression System Used
- 11.3.8. Analysis by Type of Vaccine Manufactured
- 11.3.9. Analysis by Type of Vaccine Manufactured and Location of Headquarters
12. CONCLUDING REMARKS
13. EXECUTIVE INSIGHTS
- 13.1. Chapter Overview
- 13.2. Company A
- 13.2.1. Company Snapshot
- 13.2.2. Interview Transcript: Chief Executive Officer
14. APPENDIX 1: TABULATED DATA
15. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Classification of Vaccines
- Table 3.2 Live, Attenuated Vaccines: Commonly Reported Adverse Events
- Table 3.3 Inactivated Vaccines: Commonly Reported Adverse Events
- Table 3.4 Subunit Vaccines: Commonly Reported Adverse Events
- Table 3.5 Toxoid Vaccines: Commonly Reported Adverse Events
- Table 3.6 Vaccine Excipients and their Functions
- Table 3.7 Routes of Administration and Type of Delivery Devices for Vaccine Administration
- Table 3.8 Common Pediatric Vaccines and their Routes of Administration
- Table 4.1 List of Marketed Preventive Vaccines
- Table 4.2 List of Clinical-Stage Preventive Vaccines
- Table 6.1 Preventive Vaccine Developers: List of Companies Profiled
- Table 6.2 Bio Farma: Company Overview
- Table 6.3 Bio Farma: Preventive Vaccine Pipeline
- Table 6.4 Bio Farma: Recent Developments and Future Outlook
- Table 6.5 Emergent BioSolutions: Company Overview
- Table 6.6 Emergent BioSolutions: Preventive Vaccine Pipeline
- Table 6.7 Emergent BioSolutions: Recent Developments and Future Outlook
- Table 6.8 GC Pharma: Company Overview
- Table 6.9 GC Pharma: Preventive Vaccine Pipeline
- Table 6.10 GC Pharma: Recent Developments and Future Outlook
- Table 6.11 GlaxoSmithKline: Company Overview
- Table 6.12 GlaxoSmithKline: Preventive Vaccine Pipeline
- Table 6.13 GlaxoSmithKline: Recent Developments and Future Outlook
- Table 6.14 Janssen: Company Overview
- Table 6.15 Janssen: Preventive Vaccine Pipeline
- Table 6.16 Janssen: Recent Developments and Future Outlook
- Table 6.17 Merck: Company Overview
- Table 6.18 Merck: Preventive Vaccine Pipeline
- Table 6.19 Merck: Recent Developments and Future Outlook
- Table 6.20 Novavax: Company Overview
- Table 6.21 Novavax: Preventive Vaccine Pipeline
- Table 6.22 Novavax: Recent Developments and Future Outlook
- Table 6.23 Pfizer: Company Overview
- Table 6.24 Pfizer: Preventive Vaccine Pipeline
- Table 6.25 Pfizer: Recent Developments and Future Outlook
- Table 6.26 Sanofi Pasteur: Company Overview
- Table 6.27 Sanofi Pasteur: Preventive Vaccine Pipeline
- Table 6.28 Sanofi Pasteur: Recent Developments and Future Outlook
- Table 6.29 Valneva: Company Overview
- Table 6.30 Valneva: Preventive Vaccine Pipeline
- Table 6.31 Valneva: Recent Developments and Future Outlook
- Table 8.1 Preventive Vaccines under Investigation for COVID-19
- Table 8.2 COVID-19 Vaccines: Funding Instances
- Table 8.3 Ebola Virus Disease: List of Marketed Therapeutics
- Table 8.4 Preventive Vaccines under Investigation for Ebola Virus Disease
- Table 8.5 Anti-Ebola Virus Vaccines: Funding Instances
- Table 8.6 HIV/AIDS: List of Marketed Therapeutics
- Table 8.7 Preventive Vaccines under Investigation for HIV/AIDS
- Table 8.8 Anti-HIV Vaccines: Funding Instances
- Table 8.9 Malaria: List of Marketed Therapeutics
- Table 8.10 Preventive Vaccines under Investigation for Malaria
- Table 8.11 Anti-Malaria Vaccines: Funding Instances
- Table 8.12 Preventive Vaccines under Investigation for Zika Virus Infection
- Table 8.13 Anti-Zika Virus Vaccines: Funding Instances
- Table 9.1 Preventive Vaccines: List of Funding and Investments
- Table 11.1 Vaccine Contract Manufacturers: List of Service Providers
- Table 11.2 Vaccine CMOs: Information on Type of Services Offered
- Table 11.3 Vaccine CMOs: Information on Scale of Operation
- Table 11.4 Vaccine CMOs: Information on Expression System Used
- Table 11.5 Vaccine CMOs: Information on Type of Vaccines Manufactured
- Table 11.6 List of Ad hoc Vaccine Manufacturers
- Table 12.1 Preventive Vaccines Market: Summary of the Report
- Table 14.1 Clinical-Stage Preventive Vaccines: Distribution by Type of Developer
- Table 14.2 Clinical-Stage Preventive Vaccines: Distribution by Phase of Development
- Table 14.3 Clinical-Stage Preventive Vaccines: Distribution by Route of Administration
- Table 14.4 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API
- Table 14.5 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API and Phase of Development
- Table 14.6 Clinical-Stage Preventive Vaccines: Distribution by Dosage Form
- Table 14.7 Clinical-Stage Preventive Vaccines: Distribution by Dosage
- Table 14.8 Clinical-Stage Preventive Vaccines: Distribution by Target Disease Indication
- Table 14.9 Clinical-Stage Preventive Vaccines: Distribution by Target Patient Population
- Table 14.10 Key Industry Players: Distribution by Number of Vaccines in Clinical Development
- Table 14.11 Key Non-Industry Players: Distribution by Number of Vaccines in Clinical Development
- Table 14.12 Clinical Trial Analysis: Cumulative Distribution by Trial Registration Year, Since 2010
- Table 14.13 Clinical Trial Analysis: Distribution by Number of Patients Enrolled by Trial Registration Year, Since 2010
- Table 14.14 Clinical Trial Analysis: Distribution by Trial Phase
- Table 14.15 Clinical Trial Analysis: Distribution by Trial Recruitment Status
- Table 14.16 Clinical Trial Analysis: Cumulative Year-wise Trend by Trial Recruitment Status
- Table 14.17 Clinical Trial Analysis: Distribution by Study Design
- Table 14.18 Clinical Trial Analysis: Distribution by Trial Focus Area
- Table 14.19 Clinical Trial Analysis: Distribution by Type of Preventive Vaccine (based on Pathogen)
- Table 14.20 Clinical Trial Analysis: Cumulative Year-wise Trend by Type of Preventive Vaccines
- Table 14.21 Clinical Trial Analysis: Distribution by Target Disease Indication
- Table 14.22 Clinical Trial Analysis: Distribution by Patient Enrollment and Target Disease Indication
- Table 14.23 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 14.24 Leading Industry Players: Distribution by Number of Registered Trials
- Table 14.25 Funding and Investment Analysis: Cumulative Number of Instances by Year, Since 2015
- Table 14.26 Funding and Investment Analysis: Cumulative Amount Invested, Since 2015 (USD Million)
- Table 14.27 Funding and Investment Analysis: Distribution by Type of Funding, Since 2015
- Table 14.28 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding, Since 2015 (USD Million)
- Table 14.29 Funding and Investment Analysis: Distribution of Amount Invested by Type of Vaccine API
- Table 14.30 Funding and Investment Analysis: Distribution of Amount Invested by Focus Area
- Table 14.31 Funding and Investment Analysis: Distribution of Amount Invested by Different Type of Investors
- Table 14.32 Most Active Players: Distribution by Number of Funding Instances
- Table 14.33 Most Active Investors: Distribution by Number of Funding Instances
- Table 14.34 Funding and Investment Analysis: Regional Distribution by Number of Funding Instances and Amount Raised (USD Million)
- Table 14.35 Funding and Investment Analysis: Country-wise Distribution by Total Amount Invested (USD Million)
- Table 14.36 Overall Preventive Vaccines Market, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.37 Preventive Vaccines Market, Till 2030: Distribution by Route of Administration (USD Billion)
- Table 14.38 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine (USD Billion)
- Table 14.39 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine API (USD Billion)
- Table 14.40 Preventive Vaccines Market, Till 2030: Distribution by Target Patient Population (USD Billion)
- Table 14.41 Preventive Vaccines Market, Till 2030: Distribution by Key Geographical Regions (USD Billion)
- Table 14.42 Preventive Vaccines Market in North America, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.43 Preventive Vaccines Market in the US, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.44 Preventive Vaccines Market in Mexico, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.45 Preventive Vaccines Market in Canada, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.46 Preventive Vaccines Market in Europe, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.47 Preventive Vaccines Market in Spain, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.48 Preventive Vaccines Market in the UK, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.49 Preventive Vaccines Market in Italy, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.50 Preventive Vaccines Market in France, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.51 Preventive Vaccines Market in Germany, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.52 Preventive Vaccines Market in Rest of Europe, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.53 Preventive Vaccines Market in Asia Pacific, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.54 Preventive Vaccines Market in India, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.55 Preventive Vaccines Market in China, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.56 Preventive Vaccines Market in Australia, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.57 Preventive Vaccines Market in Rest of Asia Pacific, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.58 Preventive Vaccines Market in Rest of the World, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.59 Vaccine CMOs: Distribution by Year of Establishment
- Table 14.60 Vaccine CMOs: Distribution by Company Size
- Table 14.61 Vaccine CMOs: Distribution by Scale of Operation
- Table 14.62 Vaccine CMOs: Distribution by Location of Headquarters (Region and Country-wise)
- Table 14.63 Vaccine CMOs: Distribution by Location of Manufacturing Facility (Region-wise)
- Table 14.64 Vaccine CMOs: Distribution by Type of Services Offered
- Table 14.65 Vaccine CMOs: Distribution by Expression System Used
- Table 14.66 Vaccine CMOs: Distribution by Type of Vaccines Manufactured
- Table 14.67 Vaccine CMOs: Region-wise Distribution by Type of Vaccine Manufactured
List of Figures
- Figure 3.1 Difference Between Vaccines and Small Molecules
- Figure 3.2 Classification of Vaccines
- Figure 3.3 Routes for Vaccine Administration
- Figure 4.1 Clinical-Stage Preventive Vaccines: Distribution by Type of Developer
- Figure 4.2 Clinical-Stage Preventive Vaccines: Distribution by Phase of Development
- Figure 4.3 Clinical-Stage Preventive Vaccines: Distribution by Route of Administration
- Figure 4.4 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API
- Figure 4.5 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API and Phase of Development
- Figure 4.6 Clinical-Stage Preventive Vaccines: Distribution by Dosage Form
- Figure 4.7 Clinical-Stage Preventive Vaccines: Distribution by Dosage
- Figure 4.8 Clinical-Stage Preventive Vaccines: Distribution by Target Disease Indication
- Figure 4.9 Clinical-Stage Preventive Vaccines: Distribution by Target Patient Population
- Figure 4.10 Key Industry Players: Distribution by Number of Vaccines in Clinical Development
- Figure 4.11 Key Non-Industry Players: Distribution by Number of Vaccines in Clinical Development
- Figure 5.1 Company Competitiveness Analysis: Preventive Vaccine Developers based in North America
- Figure 5.2 Company Competitiveness Analysis: Preventive Vaccine Developers based in Europe
- Figure 5.3 Company Competitiveness Analysis: Preventive Vaccine Developers based in Asia Pacific
- Figure 7.1 Clinical Trial Analysis: Scope and Methodology
- Figure 7.2 Clinical Trial Analysis: Cumulative Distribution by Trial Registration Year, Since 2010
- Figure 7.3 Clinical Trial Analysis: Distribution by Number of Patients Enrolled by Trial Registration Year, Since 2010
- Figure 7.4 Clinical Trial Analysis: Distribution by Trial Phase
- Figure 7.5 Clinical Trial Analysis: Distribution by Trial Recruitment Status
- Figure 7.6 Clinical Trial Analysis: Cumulative Year-wise Trend by Trial Recruitment Status
- Figure 7.7 Clinical Trial Analysis: Distribution by Study Design
- Figure 7.8 Clinical Trial Analysis: Distribution by Trial Focus Area
- Figure 7.9 Clinical Trial Analysis: Distribution by Type of Preventive Vaccine (based on Pathogen)
- Figure 7.10 Clinical Trial Analysis: Cumulative Year-wise Trend by Type of Preventive Vaccines
- Figure 7.11 Clinical Trial Analysis: Distribution by Target Disease Indication
- Figure 7.12 Clinical Trial Analysis: Distribution by Patient Enrollment and Target Disease Indication
- Figure 7.13 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Figure 7.14 Leading Industry Players: Distribution by Number of Registered Trials
- Figure 7.15 Clinical Trial Analysis: Geographical Distribution by Number of Registered Trials
- Figure 7.16 Clinical Trial Analysis: Geographical Distribution by Enrolled Patient Population
- Figure 7.17 Clinical Trial Analysis: Geographical Distribution by Trial Registration Year and Recruitment Status
- Figure 8.1 Historical Timeline of COVID-19 Vaccine Development
- Figure 8.2 Historical Timeline of Anti-Ebola Virus Vaccine Development
- Figure 8.3 Historical Timeline of Anti-HIV Vaccine Development
- Figure 8.4 Historical Timeline of Anti-Malaria Vaccine Development
- Figure 8.5 Historical Timeline of Anti-Zika Virus Vaccine Development
- Figure 9.1 Funding and Investment Analysis: Cumulative Number of Instances by Year, Since 2015
- Figure 9.2 Funding and Investment Analysis: Cumulative Amount Invested, Since 2015 (USD Million)
- Figure 9.3 Funding and Investment Analysis: Distribution by Type of Funding, Since 2015
- Figure 9.4 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding, Since 2015 (USD Million)
- Figure 9.5 Funding and Investment Analysis: Distribution of Amount Invested by Type of Vaccine API
- Figure 9.6 Funding and Investment Analysis: Distribution of Amount Invested and Focus Area
- Figure 9.7 Funding and Investment Analysis: Distribution of Amount Invested by Different Type of Investors
- Figure 9.8 Most Active Players: Distribution by Number of Funding Instances
- Figure 9.9 Most Active Investors: Distribution by Number of Funding Instances
- Figure 9.10 Funding and Investment Analysis: Regional Distribution by Number of Funding Instances and Amount Raised (USD Million)
- Figure 9.11 Funding and Investment Analysis: Country-wise Distribution by Total Amount Invested (USD Million)
- Figure 10.1 Overall Preventive Vaccines Market, Till 2030 (USD Billion)
- Figure 10.2 Preventive Vaccines Market, Till 2030: Distribution by Route of Administration (USD Billion)
- Figure 10.3 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine (USD Billion)
- Figure 10.4 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine API (USD Billion)
- Figure 10.5 Preventive Vaccines Market, Till 2030: Distribution by Target Patient Population (USD Billion)
- Figure 10.6 Preventive Vaccines Market, Till 2030: Distribution by Key Geographical Regions (USD Billion)
- Figure 10.7 Preventive Vaccines Market in North America, Till 2030 (USD Billion)
- Figure 10.8 Preventive Vaccines Market in the US, Till 2030 (USD Billion)
- Figure 10.9 Preventive Vaccines Market in Mexico, Till 2030 (USD Billion)
- Figure 10.10 Preventive Vaccines Market in Canada, Till 2030 (USD Billion)
- Figure 10.11 Preventive Vaccines Market in Europe, Till 2030 (USD Billion)
- Figure 10.12 Preventive Vaccines Market in Spain, Till 2030 (USD Billion)
- Figure 10.13 Preventive Vaccines Market in the UK, Till 2030 (USD Billion)
- Figure 10.14 Preventive Vaccines Market in Italy, Till 2030 (USD Billion)
- Figure 10.15 Preventive Vaccines Market in France, Till 2030 (USD Billion)
- Figure 10.16 Preventive Vaccines Market in Germany, Till 2030 (USD Billion)
- Figure 10.17 Preventive Vaccines Market in Rest of Europe, Till 2030 (USD Billion)
- Figure 10.18 Preventive Vaccines Market in Asia Pacific, Till 2030 (USD Billion)
- Figure 10.19 Preventive Vaccines Market in India, Till 2030 (USD Billion)
- Figure 10.20 Preventive Vaccines Market in China, Till 2030 (USD Billion)
- Figure 10.21 Preventive Vaccines Market in Australia, Till 2030 (USD Billion)
- Figure 10.22 Preventive Vaccines Market in Rest of Asia Pacific, Till 2030 (USD Billion)
- Figure 10.23 Preventive Vaccines Market in Rest of the World, Till 2030 (USD Billion)
- Figure 11.1 Type of Third-Party Service Providers in the Pharmaceutical Industry
- Figure 11.2 Commonly Outsourced Vaccine Development Operations
- Figure 11.3 Key Factors to Consider while Selecting a CMO Partner
- Figure 11.4 Risks and Challenges Associated with Contract Manufacturing
- Figure 11.5 Vaccine CMOs: Distribution by Year of Establishment
- Figure 11.6 Vaccine CMOs: Distribution by Company Size
- Figure 11.7 Vaccine CMOs: Distribution by Scale of Operation
- Figure 11.8 Vaccine CMOs: Distribution by Location of Headquarters (Region and Country-wise)
- Figure 11.9 Vaccine CMOs: Distribution by Location of Manufacturing Facility (Region-wise)
- Figure 11.10 Vaccine CMOs: Distribution by Type of Services Offered
- Figure 11.11 Vaccine CMOs: Distribution by Expression System Used
- Figure 11.12 Vaccine CMOs: Distribution by Type of Vaccine Manufactured
- Figure 11.13 Vaccine CMOs: Distribution by Type of Vaccine Manufactured and Location of Headquarters








