 |
市場調查報告書
商品編碼
1910846
醫藥管瓶和安瓿市佔率分析、產業趨勢與統計、成長預測(2026-2031)Pharmaceutical Glass Vials And Ampoules - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2026 - 2031) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
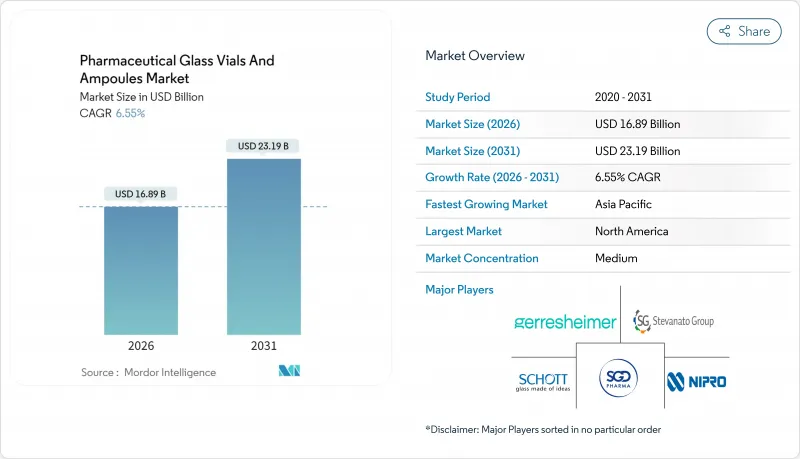
預計到 2026 年,醫藥管瓶和安瓿市場規模將達到 168.9 億美元,高於 2025 年的 158.5 億美元。預計到 2031 年,該市場規模將達到 231.9 億美元,2026 年至 2031 年的複合年成長率為 6.55%。
生物製藥產品線不斷擴展、mRNA療法對低溫運輸要求嚴格以及全球序列化強制規定等強勁基本面因素,持續推動玻璃材質優於聚合物材質。 I型硼硼矽酸玻璃憑藉其化學惰性和熱穩定性保持優勢,能夠有效保護高價值注射劑免受萃取物污染和破損。即用型(RTU)滅菌平台等技術創新降低了污染風險,縮短了填充和表面處理工程的週期,從而增強了供應商的定價權。從區域來看,亞太地區在管瓶製造領域獲得了特別顯著的資本流入,而北美則憑藉其嚴格的FDA標準保持了主導地位。市場競爭較為溫和,主要企業將投資重點放在表面塗層技術、氫氣燃燒爐和自動化視覺檢測等方面,以在成本敏感的環境中保障利潤。
全球醫藥管瓶及安瓿市場趨勢及展望
疫情後疫苗研發推動了管瓶需求
季節性疫苗宣傳活動結束後,全球疫苗研發並未放緩,多病原體疫苗計畫已擴展至呼吸道合胞病毒(RSV)、瘧疾以及聯合加強劑。為滿足多劑量製劑的需求,小容量I型管瓶的產能正在提升,這得益於諸如肖特公司(SCHOTT)11.3億美元的擴建計劃等舉措。監管機構要求從I期臨床試驗開始對最終包裝容器進行穩定性測試,將使每個項目的玻璃消費量增加約40%。兒童疫苗通常指定使用2毫升和5毫升的管瓶,這提高了對尺寸精度和嚴格顆粒閾值的要求。儘管在疫情儲備恢復正常後採購曾出現間歇性暫停,但這些趨勢共同推動了核心管瓶需求的長期成長。
生物製藥遷移到化學惰性的硼矽酸玻璃上
大分子藥物的研發管線需要耐鹼性浸出和耐表面反應的容器。 FDA於2024年發布的指南強調了相容性測試通訊協定,這實際上引導研發人員選擇I型硼硼矽酸玻璃。 Stevanato的EZ-fill平台可將萃取物含量降低至1ppm以下,這對於生物相似藥的上市而言極具閾值,因為生物類似藥的工藝可比性會受到嚴格審查。財務計算也很簡單:一次產品召回可能造成5000萬美元的成品損失,而高成本的玻璃可以有效對沖穩定性不足的風險。因此,即使聚合物容器正在蠶食低風險的通用填充市場,硼矽酸玻璃供應商仍維持高價。
聚合物管瓶正在蠶食通用玻璃容器的市場佔有率。
環烯烴聚合物容器,例如West公司的Crystal和Zenith系列產品,在診斷劑和早期生物製藥領域表現出色,因為在這些領域,抗破損性和靈活的前置作業時間比長期相容性更為訂單。考慮到搬運損耗和二次包裝,聚合物容器的單位成本優勢可達20-30%。儘管FDA的合規性障礙阻礙了聚合物在商業治療領域的應用,但在大批量、低風險的應用中,替代風險仍然是一個令人擔憂的問題。因此,標準吹製管瓶的供應商面臨著價格下降帶來的銷售壓力,促使他們進行策略轉型,轉向利潤更高的包覆和即用型(RTU)包裝。
細分市場分析
硼矽酸鹽玻璃(I型)預計將佔2025年銷售額的64.71%,這得益於其強大的監管認可度和龐大的穩定性資料庫。這種材料在製藥管瓶和安瓿瓶市場佔據主導地位,因為規避風險的製藥公司優先考慮在新應用中使用已知性能的玻璃。然而,隨著基因治療有效載荷進一步降低可萃取物容差,混合型和塗層玻璃瓶將以7.4%的複合年成長率實現最快的成長。肖特公司的Everic系列產品展示了等離子體處理表面如何減少顆粒生成,而顆粒生成這一指標正日益受到美國藥典<790>指南的嚴格審查。隨著訴訟成本飆升,採購部門正在權衡鋼化玻璃的高昂成本與生物製藥失敗造成的毀滅性損失,從而形成了一個價格彈性較低的細分市場,在這個市場中,質量比單位成本經濟性更為重要。鈉鈣玻璃(II型和III型)主要仍用於傳統的注射劑和診斷劑領域,但其市場佔有率正穩步下降,取而代之的是高等級的材料。鋁矽酸鹽混合玻璃的應用範圍較為小眾,僅限於極端熱衝擊環境的應用,例如高價值抗癌原料藥的冷凍乾燥。
展望未來五年,分析師預測硼硼矽酸玻璃將繼續保持其主導地位,同時將部分市場佔有率讓給專為高pH值病毒載體懸浮液設計的表面改性混合玻璃。供應商對氫輔助熔爐和電熔爐的投資正在縮小不同材料類別之間的碳排放強度差距,並滿足環境、社會和治理(ESG)主導的採購要求。早期採用的合約研發生產機構(CDMO)正在將容器規格諮詢納入技術轉移方案,從而在製程驗證階段有效地鎖定混合玻璃,進而為優質等級產品建立多年可預測的需求。
到2025年,疫苗將佔單位需求的45.88%,這主要得益於兒童免疫接種的持續成長和新興的旅遊健康市場。可預測的需求正推動管瓶規格向標準化瓶頸尺寸靠攏,從而促進可互換瓶塞和自動化填充線的應用。同時,生物製藥和生物相似藥將以8.09%的最高成長率成長,這主要得益於專利到期單株抗體的上市。為減少高價值治療藥物的浪費,小容量填充的趨勢正在改變藥用管瓶和安瓿的市場佔有率。即用型嵌套管式包裝與多產品生產設施中的生物製藥生產線相容,可實現更快的換型速度並提高整體設備效率(OEE)。
小分子注射劑在藥物穩定性而非包裝限制其保存期限的領域仍然具有重要意義。然而,自動注射器和預填充式注射器的日益普及正逐漸改變市場對傳統管瓶的需求。雖然胰島素得益於完善的低溫運輸體係而保持著穩定的分銷量,但持續給藥裝置的出現正在重新調整對包裝容器的需求預測。診斷試劑雖然對成本較為敏感,但由於溶劑極性和緩衝液會腐蝕聚合物容器,因此仍需要玻璃容器。這確保了即使聚合物容器技術不斷進步,診斷試劑仍能維持穩定的基準需求。
區域分析
預計到2025年,北美將佔全球收入的38.92%,這主要得益於生物製藥產能的擴張、生物製品上市核准申請(BLA)的推進,以及美國食品藥品監督管理局(FDA)優先採用I型硼矽酸玻璃的嚴格容器密封通訊協定。加拿大的聯邦生物製造舉措正在創造更多需求,並透過多年承購協議加強區域供應承諾。亞太地區的藥用玻璃管瓶和安瓿市場雖然絕對規模較小,但正以9.02%的複合年成長率快速成長,這主要得益於中國GMP規範的加強以及印度為支持熔爐現代化而生產連結獎勵計畫。韓國和新加坡的合約包裝商憑藉價格極具競爭力的即用型(RTU)產品,吸引了許多全球品牌的目光。這些產品符合ICH標準,並能縮短運往日本和澳洲的前置作業時間。
歐洲憑藉著成熟的製造商和完善的永續性框架,保持著強大的市場佔有率。然而,不斷上漲的碳權成本正在擠壓利潤空間,迫使採購部門評估混合採購模式,並利用泰國和印尼的工廠來確保供貨量。拉丁美洲受惠於美國製藥公司的近岸外包策略,尤其是在墨西哥,美墨加協定(USMCA)的貿易條款簡化了管瓶供應的海關手續。中東和非洲雖然仍處於發展初期,但具有重要的戰略意義。波灣合作理事會(GCC)成員國正在資助疫苗填充和包裝中心,並強制要求在地採購生產,這預示著該地區對初級包裝容器的新需求。整體而言,地域多元化降低了單一地區供應中斷的風險,但也迫使供應商在不同的監管環境下協調品質系統。多地點認證已成為評估提案的關鍵標準,迫使小規模的區域生產者進行合作或合併。
其他福利:
- Excel格式的市場預測(ME)表
- 3個月的分析師支持
目錄
第1章 引言
- 研究假設和市場定義
- 調查範圍
第2章調查方法
第3章執行摘要
第4章 市場情勢
- 市場概覽
- 市場促進因素
- 疫情後疫苗開發平臺推動了管瓶的需求
- 生物製藥遷移到化學惰性的硼矽酸玻璃上
- 永續性和可回收性法規賦予玻璃優勢。
- 顏色編碼安瓿瓶必須進行RFID序列化
- mRNA低溫運輸需要超低溫膨脹玻璃
- 市場限制
- 聚合物管瓶正在蠶食通用玻璃產品的市場佔有率
- 由於產品缺陷/故障導致召回次數增加,推高了風險緩解成本。
- 高pH基因治療填充劑中的鈉離子洗脫
- 高耗能爐具面臨碳定價的壓力
- 產業價值鏈分析
- 監管環境
- 技術展望
- 波特五力分析
- 供應商的議價能力
- 買方的議價能力
- 新進入者的威脅
- 替代品的威脅
- 競爭對手之間的競爭
- 宏觀經濟因素如何影響市場
第5章 市場規模與成長預測
- 依材料類型
- I型硼硼矽酸玻璃
- II/III型鈉鈣玻璃
- 鋁矽酸鹽玻璃
- 混合/表面鍍膜玻璃
- 透過使用
- 疫苗
- 胰島素
- 生物製藥和生物相似藥
- 小分子注射藥物
- 診斷劑
- 最終用戶
- 製藥公司
- 生技公司
- CDMO/CMO
- 研究和學術機構
- 醫院和診所
- 透過製造技術
- 管狀玻璃模壓
- 模壓玻璃製造
- 即用型(RTU)滅菌
- 按地區
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 南美洲
- 巴西
- 阿根廷
- 智利
- 南美洲其他地區
- 歐洲
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 其他歐洲地區
- 亞太地區
- 中國
- 日本
- 印度
- 韓國
- 澳洲
- 亞太其他地區
- 中東和非洲
- 中東
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
- 土耳其
- 其他中東地區
- 非洲
- 南非
- 奈及利亞
- 肯亞
- 其他非洲地區
- 中東
- 北美洲
第6章 競爭情勢
- 市場集中度
- 策略趨勢
- 市佔率分析
- 公司簡介
- SCHOTT AG
- Gerresheimer AG
- Stevanato Group SpA
- Nipro Corporation
- SGD SA(SGD Pharma)
- Corning Incorporated
- Bormioli Pharma SpA
- Stoelzle Oberglas GmbH
- Accu-Glass LLC
- APPL Solutions Pvt Ltd
- Shandong Pharmaceutical Glass Co., Ltd
- Chongqing Zhengchuan Pharmaceutical Packaging Co., Ltd
- Cangzhou Four Star Glass Co., Ltd
- Origin Pharma Packaging Ltd
- DWK Life Sciences GmbH
- West Pharmaceutical Services Inc.
- Sisecam Cambalkon Sanayi AS
- Stoelzle Glass Group
- Ardagh Group SA
- Beatson Clark Ltd
第7章 市場機會與未來展望
pharmaceutical glass vials and ampoules market size in 2026 is estimated at USD 16.89 billion, growing from 2025 value of USD 15.85 billion with 2031 projections showing USD 23.19 billion, growing at 6.55% CAGR over 2026-2031.

Robust fundamentals including expanding biologics pipelines, demanding cold-chain requirements for mRNA therapeutics, and global serialization mandates continue to favor glass over polymer alternatives. Type I borosilicate retains primacy because its chemical inertness and thermal stability safeguard high-value injectables from leachables and breakage. Technology upgrades such as ready-to-use (RTU) sterile platforms lower contamination risk and trim fill-finish cycle times, strengthening supplier pricing power. Regionally, Asia-Pacific registers outsized capital inflows into vial manufacturing, while North America's stringent FDA standards solidify its dominant share position. Competitive intensity remains moderate, with leaders funneling investment into surface-coating science, hydrogen-fired furnaces, and automated visual inspection to defend margins in an otherwise cost-sensitive environment.
Global Pharmaceutical Glass Vials And Ampoules Market Trends and Insights
Post-pandemic Vaccine Pipeline Boosts Vial Demand
Global vaccine development no longer tapers after seasonal campaigns; instead, multi-pathogen programs targeting RSV, malaria, and combination boosters are expanding. Capacity additions such as SCHOTT's USD 1.13 billion upgrade elevate small-volume Type I production to meet multi-dose presentation needs. Regulators insist on final-container stability testing from Phase I onward, lifting glass consumption per program by roughly 40%. Pediatric formulations often specify 2 mL and 5 mL vials, accentuating demand for dimensional accuracy and stringent particulate thresholds. These dynamics collectively reinforce a secular uplift in core vial volumes despite intermittent procurement pauses once pandemic stockpiles normalize.
Biologics Shift Toward Chemically Inert Borosilicate
Large-molecule pipelines demand containers that resist alkali leaching and surface reactivity. FDA guidance released in 2024 underscores compatibility testing protocols that implicitly steer developers toward Type I borosilicate. Stevanato's EZ-fill platform reduces extractables below 1 ppm, a threshold attractive to biosimilar launches where process comparability is scrutinized. The financial calculus is direct: a single product recall can erase USD 50 million in finished-goods value, making higher unit-price glass a rational hedge against stability failures. Consequently, borosilicate suppliers preserve premium pricing even while polymer containers nibble away at low-risk, commodity fills.
Polymer Vials Cannibalising Commodity Glass Share
Cyclic olefin polymer containers such as West's Crystal Zenith line secure orders for diagnostic reagents and early-phase biologics, where break-resistance and flexible lead times outrank lifetime compatibility. Unit economics favor polymers by 20-30% once handling losses and secondary packaging are tallied. Although FDA compatibility hurdles deter polymer uptake for commercial therapeutics, high-volume, lower-risk segments remain vulnerable to substitution. Suppliers of standard blown V-ials therefore experience volume compression at the low end, prompting a strategic pivot toward higher-margin, coated or RTU formats.
Other drivers and restraints analyzed in the detailed report include:
- Sustainability and Recyclability Regulations Favour Glass
- RFID-Serialisation Mandates for Colour-Coded Ampoules
- Fragility/Breakage Recalls Increase Risk-Mitigation Cost
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Type I borosilicate retained 64.71% of 2025 revenues, underscoring its entrenched regulatory acceptance and vast stability data bank. The pharmaceutical glass vials and ampoules market size attributed to this material outpaces all other substrates because risk-averse drug makers prioritize known performance envelopes when filing new applications. Hybrid and coated variants, however, deliver the fastest 7.4% CAGR as gene-therapy payloads push extractable allowances ever lower. SCHOTT's Everic series demonstrates how plasma-enhanced surfaces reduce particle generation, a metric increasingly scrutinized under USP <790> guidelines. As litigation costs soar, procurement teams weigh the premium of enhanced glass against the catastrophic downside of biologic batch failures, creating a price-in-elastic niche where quality trumps unit economics. Soda-lime glass-types II and III-survive mostly in legacy, small-molecule injectables and diagnostic reagents, but their share steadily erodes in favor of higher-grade materials. Aluminum-silicate formulations remain niche, reserved for extreme thermal-shock scenarios such as freeze-drying of high-value oncology APIs.
Across a five-year horizon, analysts expect borosilicate to retain a majority stake yet cede incremental share to surface-engineered hybrids designed for high-pH viral vector suspensions. Supplier investments in hydrogen-assisted furnaces and electric melting reduce the carbon intensity gap between material classes, accommodating ESG-driven sourcing mandates. Early-adopter CDMOs are bundling container specification counseling into tech-transfer packages, effectively locking in hybrid glass at the process-validation stage and cementing multiyear demand visibility for premium grades.
Vaccines accounted for 45.88% of 2025 unit demand, undergirded by ongoing pediatric immunization and emerging travel-health indications. Given volume predictability, vial formats have converged on standardized neck dimensions facilitating interchangeable stoppers and automated filling lines. Meanwhile, biologics and biosimilars claim the highest 8.09% growth trajectory, fueled by monoclonal antibody launches post-patent cliff. Here, the pharmaceutical glass vials and ampoules market share shifts toward smaller fill volumes that mitigate wastage for high-price therapies. RTU nest-and-tub formats resonate with biologics lines operating in multiproduct facilities, offering rapid changeovers that boost overall equipment effectiveness.
Small-molecule injectables preserve relevance where drug stability, not packaging, constrains shelf life; however, rising adoption of auto-injectors and prefilled syringes gradually siphons volume from traditional vials. Insulin maintains steady throughput thanks to entrenched cold-chain infrastructure, but continuous-delivery devices are beginning to recalibrate container demand forecasts. Diagnostic reagents, although cost-sensitive, continue to specify glass where solvent polarity or buffered media attack polymer walls, ensuring a residual baseline volume even amid polymer advances.
The Pharmaceutical Glass Vials and Ampoules Market Report is Segmented by Material Type (Type I Borosilicate Glass, Type II/III Soda-Lime Glass and More), Application (Vaccines, Insulin and More), End User (Pharmaceutical Manufacturers, Biotechnology Companies and More), Manufacturing Technology (Tubular Glass Forming, Moulded Glass Forming and More), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained 38.92% of 2025 revenues, buoyed by expansive biologics capacity, Biologics License Application pipelines, and the FDA's strict container-closure protocols that privilege Type I borosilicate. Canada's federal bio-manufacturing initiative adds incremental demand, reinforcing regional supply commitments with multi-year offtake agreements. The pharmaceutical glass vials and ampoules market size in Asia-Pacific, while smaller in absolute terms, grows at a 9.02% CAGR on the back of Chinese GMP enhancements and Indian production-linked incentives that subsidize furnace modernization. Contract packagers in South Korea and Singapore lure global brands with competitively priced RTU offerings that meet ICH standards, trimming lead times into Japan and Australia.
Europe commands robust share underpinned by legacy manufacturers and a strong sustainability framework; yet rising carbon-credit costs pressure margins, nudging procurement to evaluate mixed sourcing models tapping Thai and Indonesian plants for commodity volumes. Latin America benefits from near-shoring strategies by U.S. pharma, particularly in Mexico where USMCA trade provisions smooth customs hurdles for vial supply. The Middle East and Africa remain nascent but strategic, with Gulf Cooperation Council nations funding vaccine fill-finish hubs that stipulate local content thresholds, foreshadowing fresh regional demand for primary containers. Collectively, geographic diversification mitigates single-region disruption risk, but it forces suppliers to harmonize quality systems across heterogeneous regulatory landscapes. Multisite qualification emerges as a decisive criterion in request-for-proposal scoring, pushing small regional producers to partner or consolidate.
- SCHOTT AG
- Gerresheimer AG
- Stevanato Group S.p.A.
- Nipro Corporation
- SGD S.A. (SGD Pharma)
- Corning Incorporated
- Bormioli Pharma S.p.A.
- Stoelzle Oberglas GmbH
- Accu-Glass LLC
- APPL Solutions Pvt Ltd
- Shandong Pharmaceutical Glass Co., Ltd
- Chongqing Zhengchuan Pharmaceutical Packaging Co., Ltd
- Cangzhou Four Star Glass Co., Ltd
- Origin Pharma Packaging Ltd
- DWK Life Sciences GmbH
- West Pharmaceutical Services Inc.
- Sisecam Cambalkon Sanayi A.S.
- Stoelzle Glass Group
- Ardagh Group S.A.
- Beatson Clark Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Post-pandemic vaccine pipeline boosts vial demand
- 4.2.2 Biologics shift toward chemically inert borosilicate
- 4.2.3 Sustainability and recyclability regulations favour glass
- 4.2.4 RFID-serialisation mandates for colour-coded ampoules
- 4.2.5 mRNA cold-chain needs ultra-low expansion glass
- 4.3 Market Restraints
- 4.3.1 Polymer vials cannibalising commodity glass share
- 4.3.2 Fragility/breakage recalls increase risk-mitigation cost
- 4.3.3 Sodium-ion leaching in high-pH gene-therapy fills
- 4.3.4 Energy-intensive furnaces face carbon-pricing pressure
- 4.4 Industry Value Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porters Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
- 4.8 Impact of Macroeconomic Factors on the Market
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Material Type
- 5.1.1 Type I Borosilicate Glass
- 5.1.2 Type II/III Soda-Lime Glass
- 5.1.3 Aluminum-Silicate Glass
- 5.1.4 Hybrid / Surface-Coated Glass
- 5.2 By Application
- 5.2.1 Vaccines
- 5.2.2 Insulin
- 5.2.3 Biologics and Biosimilars
- 5.2.4 Small-Molecule Injectables
- 5.2.5 Diagnostic Reagents
- 5.3 By End User
- 5.3.1 Pharmaceutical Manufacturers
- 5.3.2 Biotechnology Companies
- 5.3.3 CDMOs / CMOs
- 5.3.4 Research and Academic Laboratories
- 5.3.5 Hospitals and Clinics
- 5.4 By Manufacturing Technology
- 5.4.1 Tubular Glass Forming
- 5.4.2 Moulded Glass Forming
- 5.4.3 Ready-to-Use (RTU) Sterile
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 South America
- 5.5.2.1 Brazil
- 5.5.2.2 Argentina
- 5.5.2.3 Chile
- 5.5.2.4 Rest of South America
- 5.5.3 Europe
- 5.5.3.1 Germany
- 5.5.3.2 United Kingdom
- 5.5.3.3 France
- 5.5.3.4 Italy
- 5.5.3.5 Spain
- 5.5.3.6 Rest of Europe
- 5.5.4 Asia-Pacific
- 5.5.4.1 China
- 5.5.4.2 Japan
- 5.5.4.3 India
- 5.5.4.4 South Korea
- 5.5.4.5 Australia
- 5.5.4.6 Rest of Asia-Pacific
- 5.5.5 Middle East and Africa
- 5.5.5.1 Middle East
- 5.5.5.1.1 Saudi Arabia
- 5.5.5.1.2 United Arab Emirates
- 5.5.5.1.3 Turkey
- 5.5.5.1.4 Rest of Middle East
- 5.5.5.2 Africa
- 5.5.5.2.1 South Africa
- 5.5.5.2.2 Nigeria
- 5.5.5.2.3 Kenya
- 5.5.5.2.4 Rest of Africa
- 5.5.5.1 Middle East
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global Level Overview, Market Level Overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, Recent Developments)
- 6.4.1 SCHOTT AG
- 6.4.2 Gerresheimer AG
- 6.4.3 Stevanato Group S.p.A.
- 6.4.4 Nipro Corporation
- 6.4.5 SGD S.A. (SGD Pharma)
- 6.4.6 Corning Incorporated
- 6.4.7 Bormioli Pharma S.p.A.
- 6.4.8 Stoelzle Oberglas GmbH
- 6.4.9 Accu-Glass LLC
- 6.4.10 APPL Solutions Pvt Ltd
- 6.4.11 Shandong Pharmaceutical Glass Co., Ltd
- 6.4.12 Chongqing Zhengchuan Pharmaceutical Packaging Co., Ltd
- 6.4.13 Cangzhou Four Star Glass Co., Ltd
- 6.4.14 Origin Pharma Packaging Ltd
- 6.4.15 DWK Life Sciences GmbH
- 6.4.16 West Pharmaceutical Services Inc.
- 6.4.17 Sisecam Cambalkon Sanayi A.S.
- 6.4.18 Stoelzle Glass Group
- 6.4.19 Ardagh Group S.A.
- 6.4.20 Beatson Clark Ltd
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-Space and Unmet-Need Assessment










