 |
市場調查報告書
商品編碼
1851544
醫療保健領域的印刷電子技術:市場佔有率分析、行業趨勢、統計數據和成長預測(2025-2030 年)Printed Electronics In Healthcare - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
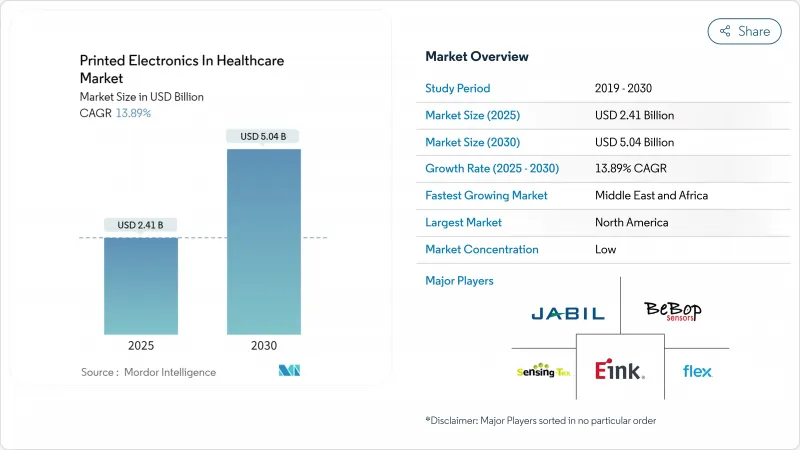
目前醫療保健領域印刷電子產品的市場規模為 24.1 億美元,預計到 2030 年將達到 50.4 億美元,複合年成長率為 13.89%。
這項強勁成長得益於該技術能夠以傳統矽製造製程無法比擬的單位成本,提供軟性、輕巧且一次性使用的醫療設備。對用於遠端患者監護的穿戴式設備的強勁需求、智慧藥品包裝的成長以及生物相容性導電油墨的快速創新,都為近期成長提供了支撐。北美地區監管政策的早期明確和美國國立衛生研究院 (NIH) 的慷慨津貼將加速商業化進程,而亞太地區對照護現場的推動將擴大基本客群。同時,隨著臨床應用障礙的克服,自修復導體和可拉伸基板的突破有望帶來新的收入來源。
全球醫療保健印刷電子市場趨勢與洞察
美國居家醫療領域迅速採用穿戴貼片進行遠端患者監護
遠端醫療)擴大了遠距醫療的報銷範圍,美國食品藥物管理局(FDA)批准了親膚型血糖貼片和心臟貼片,這些措施正推動印刷感測器在居家醫療領域的廣泛應用。混合微流控貼片現在可以收集多參數生命徵象,為醫療團隊提供詳細的縱向數據,無需患者前往診所。美國醫療系統報告稱,再入院率降低,病患滿意度提高,證實了實質的成本節約。在此環境下,設備製造商的規模化生產為歐盟和亞太地區的醫療系統在評估報銷框架時樹立了令人信服的先例。
歐盟打擊假藥指令推動採用印刷式射頻辨識技術的智慧藥品包裝。
歐盟《反假藥指令》的全面序列化要求迫使製藥公司在每個零售包裝中加入防偽功能。採用高速柔版印刷生產線生產的印刷式RFID和NFC標籤,如今既滿足了可追溯性要求,又具備防篡改功能,且單位成本達到非專利和品牌藥生產商可接受的水平。採用符合歐盟標準的包裝的全球製藥公司,也正將同樣的解決方案推廣到亞太地區的物流中心,從而對適用於紙張和箔材的導電油墨的需求產生了倍增效應。
FDA和EMA的檢驗週期延緩了商業推廣。
實際的510(k)審查通常遠超預期,耗時六至七個月,因為審查人員會要求提供新基材的額外實驗室和臨床數據。此外,歐洲新的醫療器材法規(MDR)增加了臨床性能測試,迫使企業提交雙軌申請。網路安全和人工智慧文件要求的提高以及測試成本的增加,導致一些中型企業推遲在美國上市,轉而選擇在中東和南美進行試點部署。
細分市場分析
到2024年,印刷生物感測器將佔印刷電子市場41.8%的佔有率。血糖試紙和連續血糖監測儀將佔據市場主導地位,這得益於美國市場核准的通過,使得常規血糖監測不再那麼令人詬病。感染疾病檢測將繼續成為亞太地區公共衛生競標的主要成長引擎,而新興的pH貼片和傷口監測貼片也正在獲得臨床應用。
可拉伸軟性混合電子產品將成為同類產品中成長最快的,複合年成長率將達到16.3%。自修復導電網能夠承受反覆的應變循環而不發生分層,從而實現長達一週的心臟和神經系統監測。用於藥品包裝的印刷型RFID標籤將成為第二大需求支柱,尤其是在全球序列化要求日益成熟的背景下。
由於網版印刷技術具有成熟的生產效率和一次性電極的低單位成本,預計到2024年,它將佔據印刷電子市場52.9%的佔有率。成熟的製程控制有助於通過FDA認證,使其成為大規模生產生物感測器的首選製程。
氣溶膠噴射和3D列印技術正以14.7%的複合年成長率成長。在微流體通道內沉積導電線的能力,已將原型製作時間從數天縮短至數分鐘。瑞士和新加坡的早期採用者已在試點規模上實現了亞100微米通道的精度,從而能夠快速迭代實驗室晶片診斷設計。
區域分析
2024年,北美將佔全球銷售額的40.8%,這得益於FDA早期推出的數位健康框架和NIH的資助計劃,這些計劃降低了材料研發的風險。梅奧診所和克利夫蘭診所進行的多中心試驗檢驗了遠端監測終點,並簡化了區域醫院網路的採購核准。位於安大略省的加拿大研究叢集在特種基板累積了豐富的專業知識,進一步鞏固了我們在北美的領先地位。
歐洲仍是重要的戰略要地。歐洲的大型製藥公司必須遵守《反假藥指令》,這持續催生了對序列化智慧標籤的需求。德國的精密機械製造傳統為大批量印刷機提供了支持,而英國則將風險投資投入創業投資資金靈活的新興企業新創公司。法國和北歐國家的公共衛生部門將透過遠端感測器的預防性醫療報銷來鼓勵其廣泛應用。
預計中東和非洲地區的複合年成長率將達到15.4%,位居全球之首。沙烏地阿拉伯和阿拉伯聯合大公國的國家衛生推廣計畫已為連網診斷技術撥出預算,將印刷電子技術視為無需龐大基礎設施即可快速覆蓋農村地區的途徑。南非監管機構正將醫療器材代碼與美國食品藥物管理局(FDA)的分類標準接軌,加速進口核准。這一勢頭標誌著該地區在醫療技術自主化方面又向前邁進了一步。
其他福利:
- Excel格式的市場預測(ME)表
- 3個月的分析師支持
目錄
第1章 引言
- 研究假設和市場定義
- 調查範圍
第2章調查方法
第3章執行摘要
第4章 市場情勢
- 市場概覽
- 市場促進因素
- 美國居家醫療領域對穿戴式遠距病人監護貼片的快速採用
- 歐盟假藥指令推廣採用印有RFID技術的智慧藥品包裝。
- 用於感染疾病檢測的照護現場檢測一次性生物感測器在亞洲迅速普及。
- 慢性疾病負擔推動了心臟病學領域對軟性印刷電極的需求。
- 為保障低溫運輸完整性,疫苗領域亟需採用印刷式溫度感測器。
- 由美國國立衛生研究院和歐盟地平線津貼資助的生物相容性導電油墨研發
- 市場限制
- FDA和EMA的檢驗週期延緩了商業推廣。
- 聚合物基材的滅菌和生物相容性挑戰
- 濕度劣化會降低印刷生物感測器的使用壽命。
- 新興市場穿戴診斷報銷的不確定性
- 生態系分析
- 監理與技術展望
- 印刷技術概覽
- 網版印刷
- 噴墨列印
- 凹版印刷及柔版印刷
- 氣溶膠噴射和其他新興技術
- 印刷技術概覽
- 波特五力分析
- 新進入者的威脅
- 買方的議價能力
- 供應商的議價能力
- 替代品的威脅
- 競爭對手之間的競爭
第5章 市場規模與成長預測
- 按類型
- 印刷生物感測器
- 葡萄糖感測器
- 感染疾病檢測條
- 其他生物感測器
- 印刷式生理感測器
- 心電圖/腦電圖電極
- 溫度/pH貼片
- 印刷型RFID/NFC標籤
- 伸縮性軟性混合電子裝置
- 印刷微射流
- 其他列印零件(天線、加熱器)
- 印刷生物感測器
- 透過印刷技術
- 網版印刷
- 噴墨列印
- 凹版/柔版
- 氣溶膠噴射和3D列印
- 透過使用
- 病患監測和穿戴式設備
- 診斷檢測和照護現場
- 藥物傳輸和智慧型貼片
- 藥品包裝和仿冒品
- 醫療影像和治療設備
- 其他
- 最終用戶
- 醫院和診所
- 家庭醫療保健提供者
- 製藥和生物技術公司
- 診斷實驗室
- 學術研究機構
- 按地區
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 英國
- 法國
- 北歐國家
- 其他歐洲地區
- 南美洲
- 巴西
- 南美洲其他地區
- 亞太地區
- 中國
- 日本
- 印度
- 東南亞
- 亞太其他地區
- 中東和非洲
- 中東
- GCC
- 土耳其
- 其他中東地區
- 非洲
- 南非
- 其他非洲地區
- 北美洲
第6章 競爭情勢
- 市場集中度
- 策略趨勢
- 市佔率分析
- 公司簡介
- DuPont de Nemours, Inc.
- Henkel AG and Co. KGaA
- Nissha Co., Ltd.(Nissha Medical)
- Flex Ltd.(Health Solutions)
- Molex LLC(Sensable)
- GE Healthcare
- Abbott Laboratories(FreeStyle Libre Sensors)
- Medtronic plc(Printed Electrodes)
- Jabil Inc.(Blue Sky Center)
- Zimmer Biomet(Smart Implants)
- NovaCentrix Corp.
- PragmatIC Semiconductor Ltd.
- Thinfilm Electronics ASA
- Toppan Printing Co., Ltd.(Healthcare Labels)
- PolyIC GmbH and Co. KG
- GSI Technologies LLC
- PV Nano Cell Ltd.
- Coatema Coating Machinery GmbH
- Bebop Sensors Inc.
- Sensing Tex SL
第7章 市場機會與未來展望
The printed electronics market size in healthcare is currently valued at USD 2.41 billion and is forecast to achieve USD 5.04 billion by 2030, reflecting a 13.89% CAGR.

This vigorous expansion stems from the technology's ability to deliver flexible, lightweight, and disposable medical devices at unit costs traditional silicon manufacturing cannot match. Strong demand for remote patient-monitoring wearables, growth in smart pharmaceutical packaging, and rapid innovation in biocompatible conductive inks anchor near-term growth. North America's early regulatory clarity and generous NIH grants accelerate commercialization pipelines, while Asia-Pacific's push for point-of-care diagnostics widens the customer base. Meanwhile, breakthroughs in self-healing conductors and stretchable substrates promise fresh revenue streams as clinical adoption hurdles are cleared.
Global Printed Electronics In Healthcare Market Trends and Insights
Rapid Uptake of Remote Patient-Monitoring Wearable Patches in United States Homecare
Medicare's broader reimbursement for telehealth, coupled with FDA clearance of skin-friendly glucose and cardiac patches, fuels wide deployment of printed sensors in the home-care channel. Hybrid microfluidic-regulated patches now capture multi-parameter vitals, handing care teams granular longitudinal data without clinic visits. U.S. systems report fewer readmissions and higher patient satisfaction, confirming tangible cost savings. Device makers scaling in this environment set a compelling precedent for EU and APAC health systems as they evaluate reimbursement frameworks.
EU Falsified Medicines Directive Catalyzing Smart Pharma-Packaging with Printed RFID
Full serialization under the EU Falsified Medicines Directive forces pharmaceutical producers to embed authentication features on every retail pack. Printed RFID and NFC tags, fabricated on high-speed flexographic lines, now satisfy both traceability and tamper evidence at unit cost levels acceptable to generic and branded manufacturers. Global drug firms adopting EU-compliant packaging extend the same solutions to APAC logistics hubs, creating a multiplier effect on demand for conductive inks optimized for paper and foil substrates.
FDA and EMA Validation Cycles Delaying Commercial Roll-outs
Actual 510(k) reviews often stretch to 6-7 months, well beyond nominal timelines, as examiners request extra bench and clinical data on novel substrates. De novo classifications lengthen approvals further, and the new European MDR imposes additional clinical performance studies, forcing dual submission tracks. Rising cybersecurity and AI documentation requirements add layers of testing cost, prompting some mid-cap firms to defer U.S. launches in favor of pilot deployments in MEA or South America.
Other drivers and restraints analyzed in the detailed report include:
- Surge in Point-of-Care Disposable Biosensors for Infectious Disease Detection in Asia
- Cold-Chain Integrity Needs Boosting Printed Temperature Sensors for Vaccines
- Sterilization and Biocompatibility Challenges of Polymer Substrates
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Printed biosensors held 41.8% of the printed electronics market in 2024. Glucose strips and continuous glucose monitors dominate the installed base, buoyed by over-the-counter U.S. approvals that destigmatize routine monitoring. Infectious disease assays remain a growth engine in APAC public-health tenders, while emerging pH and wound-monitor patches broaden clinical reach.
Stretchable and flexible hybrid electronics is projected to post a 16.3% CAGR, the fastest among types. Self-healing conductive meshes now survive repeated strain cycles without delamination, allowing week-long cardiac or neuro monitoring. Printed RFID labels for pharma packs add a second demand pillar, especially as global serialization mandates mature
Screen printing captured 52.9% of the printed electronics market in 2024 thanks to proven throughput and low per-unit costs for disposable electrodes. Mature process controls ease FDA filing, making it the default for high-volume biosensors.
Aerosol jet and 3D methods are growing at a 14.7% CAGR. Their ability to deposit conductive tracks inside 3D microfluidic channels has cut prototyping times from days to minutes. Early adopters in Switzerland and Singapore have demonstrated sub-100 µm channel fidelity at pilot scale, enabling rapid design iteration for lab-on-chip diagnostics.
The Printed Electronics in Healthcare Market Report is Segmented by Type (Printed Biosensors, Printed Physiological Sensors, Printed RFID/NFC Labels, and More), Printing Technology (Screen Printing, Inkjet Printing, and More), Application (Patient Monitoring, Diagnostic Testing, Drug Delivery, and More), End-User (Hospitals, Home Healthcare, and More), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America posted 40.8% of global revenue in 2024, benefiting from FDA's early digital-health frameworks and NIH funding streams that de-risk material R&D. Multicenter trials at Mayo Clinic and Cleveland Clinic validate remote monitoring endpoints, smoothing procurement approvals for regional hospital networks. Canadian research clusters in Ontario add specialized substrate expertise, further bolstering continental leadership.
Europe remains a strategic stronghold. The region's pharmaceutical giants must comply with the Falsified Medicines Directive, locking in sustained demand for serialized smart tags. Germany's precision-machinery heritage supports high-volume printing presses, while the United Kingdom channels venture funding into flexible IC startups. Public health authorities in France and Nordic nations augment uptake through preventive-care reimbursement for remote sensors.
The Middle East and Africa is forecast to deliver a 15.4% CAGR, the fastest worldwide. National health expansions in Saudi Arabia and the United Arab Emirates allocate budget lines for connected diagnostics, seeing printed electronics as a quick path to rural coverage without heavy infrastructure. South Africa's regulatory agency aligns its device code to FDA classification, accelerating import approvals. This momentum signals a step-change in the region's medical technology self-sufficiency.
- DuPont de Nemours, Inc.
- Henkel AG and Co. KGaA
- Nissha Co., Ltd. (Nissha Medical)
- Flex Ltd. (Health Solutions)
- Molex LLC (Sensable)
- GE Healthcare
- Abbott Laboratories (FreeStyle Libre Sensors)
- Medtronic plc (Printed Electrodes)
- Jabil Inc. (Blue Sky Center)
- Zimmer Biomet (Smart Implants)
- NovaCentrix Corp.
- PragmatIC Semiconductor Ltd.
- Thinfilm Electronics ASA
- Toppan Printing Co., Ltd. (Healthcare Labels)
- PolyIC GmbH and Co. KG
- GSI Technologies LLC
- PV Nano Cell Ltd.
- Coatema Coating Machinery GmbH
- Bebop Sensors Inc.
- Sensing Tex S.L.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid Uptake of Remote Patient-Monitoring Wearable Patches in United States Homecare
- 4.2.2 EU Falsified Medicines Directive Catalyzing Smart Pharma-Packaging with Printed RFID
- 4.2.3 Surge in Point-of-Care Disposable Biosensors for Infectious Disease Detection in Asia
- 4.2.4 Chronic-Disease Burden Driving Demand for Flexible Printed Electrodes in Cardiology
- 4.2.5 Cold-Chain Integrity Needs Boosting Printed Temperature Sensors for Vaccines
- 4.2.6 NIH and EU Horizon Grants Funding Bio-Compatible Conductive-Ink R&D
- 4.3 Market Restraints
- 4.3.1 FDA and EMA Validation Cycles Delaying Commercial Roll-outs
- 4.3.2 Sterilization and Biocompatibility Challenges of Polymer Substrates
- 4.3.3 Humidity-Driven Degradation Limiting Shelf-Life of Printed Biosensors
- 4.3.4 Reimbursement Ambiguity for Wearable Diagnostics in Emerging Markets
- 4.4 Industry Ecosystem Analysis
- 4.5 Regulatory and Technological Outlook
- 4.5.1 Printing Technology Snapshot
- 4.5.1.1 Screen Printing
- 4.5.1.2 Inkjet Printing
- 4.5.1.3 Gravure and Flexography
- 4.5.1.4 Aerosol Jet and Other Emerging Techniques
- 4.5.1 Printing Technology Snapshot
- 4.6 Porter's Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitute Products
- 4.6.5 Intensity of Competitive Rivalry
5 MARKET SIZE AND GROWTH FORECASTS (VALUES)
- 5.1 By Type
- 5.1.1 Printed Biosensors
- 5.1.1.1 Glucose Sensors
- 5.1.1.2 Infectious-Disease Test Strips
- 5.1.1.3 Other Biosensors
- 5.1.2 Printed Physiological Sensors
- 5.1.2.1 ECG/EEG Electrodes
- 5.1.2.2 Temperature/pH Patches
- 5.1.3 Printed RFID/NFC Labels
- 5.1.4 Stretchable and Flexible Hybrid Electronics
- 5.1.5 Printed Microfluidics
- 5.1.6 Other Printed Components (Antennas, Heaters)
- 5.1.1 Printed Biosensors
- 5.2 By Printing Technology
- 5.2.1 Screen Printing
- 5.2.2 Inkjet Printing
- 5.2.3 Gravure/Flexography
- 5.2.4 Aerosol Jet and 3D Printing
- 5.3 By Application
- 5.3.1 Patient Monitoring and Wearables
- 5.3.2 Diagnostic Testing and Point-of-Care
- 5.3.3 Drug Delivery and Smart Patches
- 5.3.4 Pharmaceutical Packaging and Anti-Counterfeit
- 5.3.5 Medical Imaging and Therapeutic Devices
- 5.3.6 Others
- 5.4 By End-user
- 5.4.1 Hospitals and Clinics
- 5.4.2 Home Healthcare Providers
- 5.4.3 Pharmaceutical and Biotech Companies
- 5.4.4 Diagnostic Laboratories
- 5.4.5 Academic and Research Institutes
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Nordics
- 5.5.2.5 Rest of Europe
- 5.5.3 South America
- 5.5.3.1 Brazil
- 5.5.3.2 Rest of South America
- 5.5.4 Asia-Pacific
- 5.5.4.1 China
- 5.5.4.2 Japan
- 5.5.4.3 India
- 5.5.4.4 South-East Asia
- 5.5.4.5 Rest of Asia-Pacific
- 5.5.5 Middle East and Africa
- 5.5.5.1 Middle East
- 5.5.5.1.1 Gulf Cooperation Council Countries
- 5.5.5.1.2 Turkey
- 5.5.5.1.3 Rest of Middle East
- 5.5.5.2 Africa
- 5.5.5.2.1 South Africa
- 5.5.5.2.2 Rest of Africa
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 DuPont de Nemours, Inc.
- 6.4.2 Henkel AG and Co. KGaA
- 6.4.3 Nissha Co., Ltd. (Nissha Medical)
- 6.4.4 Flex Ltd. (Health Solutions)
- 6.4.5 Molex LLC (Sensable)
- 6.4.6 GE Healthcare
- 6.4.7 Abbott Laboratories (FreeStyle Libre Sensors)
- 6.4.8 Medtronic plc (Printed Electrodes)
- 6.4.9 Jabil Inc. (Blue Sky Center)
- 6.4.10 Zimmer Biomet (Smart Implants)
- 6.4.11 NovaCentrix Corp.
- 6.4.12 PragmatIC Semiconductor Ltd.
- 6.4.13 Thinfilm Electronics ASA
- 6.4.14 Toppan Printing Co., Ltd. (Healthcare Labels)
- 6.4.15 PolyIC GmbH and Co. KG
- 6.4.16 GSI Technologies LLC
- 6.4.17 PV Nano Cell Ltd.
- 6.4.18 Coatema Coating Machinery GmbH
- 6.4.19 Bebop Sensors Inc.
- 6.4.20 Sensing Tex S.L.
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-space and Unmet-Need Assessment












