 |
市場調查報告書
商品編碼
1793323
全球創傷護理市場(按產品、傷口、最終用戶和地區分類)- 預測至 2030 年Wound Care Market by Product (Dressings, Devices, Biological Skin Substitutes, Sutures, Staplers), By Wounds, By End User, and Region -Global Forecast to 2030 |
||||||
預計全球創傷護理市場將從 2025 年的 222.2 億美元成長到 2030 年的 304.8 億美元,預測期內的複合年成長率為 6.5%。
| 調查範圍 | |
|---|---|
| 調查年份 | 2024-2030 |
| 基準年 | 2024 |
| 預測期 | 2025-2030 |
| 單元 | 10億美元 |
| 部分 | 產品、傷口類型、最終用戶 |
| 目標區域 | 北美、歐洲、亞太地區、拉丁美洲、中東和非洲 |
糖尿病患者數量不斷增加、創傷和燒傷病例不斷增加以及老年人口不斷成長,這些因素推動了市場的成長。此外,先進創傷護理產品的相關風險也限制市場的成長。
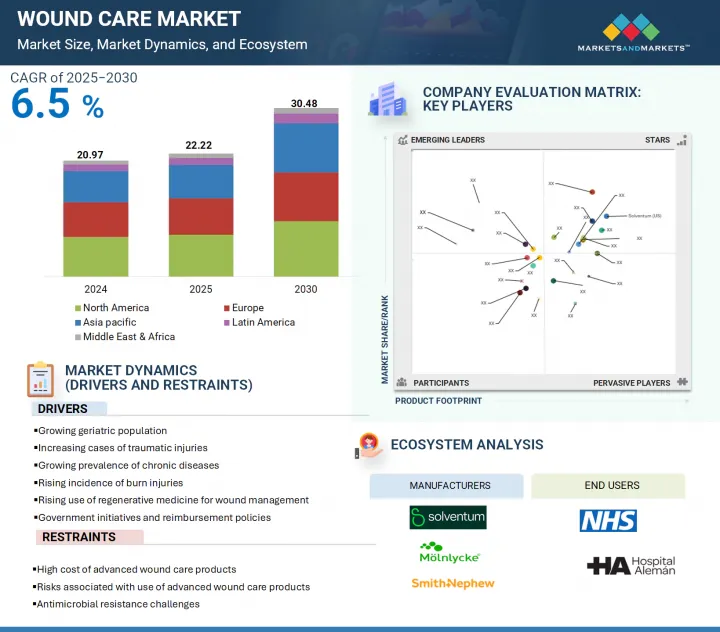
根據最終用戶,預計創傷護理市場的醫院和診所部分將在預測期內佔據最大地位。
到2024年,醫院和診所將佔據最大的市場佔有率。這種主導地位的驅動力在於糖尿病足潰瘍和手術傷口的盛行率不斷上升,這些疾病需要專門的治療。此外,患有糖尿病和血管疾病等慢性疾病的老年人也擴大前往醫院進行創傷護理。醫院和專科護理中心擁有經驗豐富的醫務人員、標準化的治療方案以及種類繁多的創傷護理產品,從而能夠高效有效地進行治療。持續關注改善創傷護理效果和患者康復,進一步鞏固了該領域的主導地位。
亞太地區是創傷護理市場成長最快的地區。
由於老年人口不斷成長以及導致慢性傷口的文明病的增多,亞太地區創傷護理市場正在不斷擴張。對優質醫療服務的需求不斷成長,以及對醫院基礎設施和醫療技術的投資不斷增加,推動了先進創傷護理產品的普及。此外,印度、泰國和新加坡等國醫療旅遊業的蓬勃發展,加上手術數量的增加,進一步推動了術後創傷護理的需求。由於亞太地區的醫療保健提供者專注於改善治療效果並縮短康復時間,預計該地區將迎來強勁成長,預計在預測期內,該地區的複合年成長率最高。
本報告分析了全球創傷護理市場,提供了關鍵促進因素和限制因素、競爭格局和未來趨勢的資訊。
目錄
第1章 引言
第2章調查方法
第3章執行摘要
第4章 主要發現
- 創傷護理市場概況
- 亞太地區創傷護理市場佔有率
- 創傷護理市場(按主要國家分類)
- 創傷護理市場:區域構成(2023-2030)
第5章市場概述
- 介紹
- 市場動態
- 驅動程式
- 抑制因素
- 機會
- 任務
- 影響客戶業務的趨勢/中斷
- 定價分析
- 平均售價趨勢:按產品
- 各主要傷口類型及主要企業創傷護理產品平均售價趨勢
- 各地區平均銷售價格趨勢
- 價值鏈分析
- 供應鏈分析
- 生態系分析
- 投資金籌措場景
- 技術分析
- 主要技術
- 互補技術
- 鄰近技術
- 專利分析
- 貿易分析
- 導入場景
- 匯出場景
- 大型會議和活動(2025-2026年)
- 案例研究分析
- 案例研究1:將先進的創傷護理策略應用於複雜的創傷治療
- 案例研究2:新型親水膠體敷料在創傷護理中的評估
- 案例研究3:使用 COLOPLAST 開發的簡單三步驟框架支持難治性壓瘡的自我管理
- 監管格局
- 監管分析
- 監管機構、政府機構和其他組織
- 波特五力分析
- 主要相關利益者和採購標準
- 人工智慧對創傷護理市場的影響
- 介紹
- 人工智慧在創傷護理的市場潛力
- 人工智慧對創傷護理市場的影響:用例
- 採用人工智慧的主要企業
- 人工智慧在創傷護理市場的未來
- 2025年美國關稅
- 介紹
- 主要關稅稅率
- 價格影響分析
- 對國家的影響
- 對終端產業的影響
第6章創傷護理市場(按產品)
- 介紹
- 高級創傷護理產品
- 手術創傷護理產品
- 傳統創傷護理產品
第7章創傷護理市場(依傷口類型)
- 介紹
- 慢性傷口
- 急性傷口
第8章創傷護理市場(按最終用戶)
- 介紹
- 醫院和診所
- 居家照護環境
- 長期照護機構
- 其他最終用戶
第9章創傷護理市場(按地區)
- 介紹
- 北美洲
- 北美宏觀經濟展望
- 美國
- 加拿大
- 歐洲
- 歐洲宏觀經濟展望
- 德國
- 法國
- 英國
- 義大利
- 西班牙
- 俄羅斯
- 其他歐洲國家
- 亞太地區
- 亞太宏觀經濟展望
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 新加坡
- 其他亞太地區
- 拉丁美洲
- 拉丁美洲宏觀經濟展望
- 巴西
- 墨西哥
- 其他拉丁美洲
- 中東和非洲
第10章 競爭格局
- 概述
- 主要參與企業的策略/優勢(2,024)
- 收益分析(2022-2024)
- 市場佔有率分析(2024年)
- 企業評估矩陣:主要企業(2024年)
- 公司評估矩陣:Start-Ups/中小企業(2024 年)
- 公司估值及財務指標
- 品牌/產品比較
- 競爭場景
第11章 公司簡介
- 主要企業
- SOLVENTUM
- JOHNSON & JOHNSON SERVICES, INC. (ETHICON)
- SMITH+NEPHEW
- CARDINAL HEALTH
- MOLNLYCKE AB
- CONVATEC GROUP PLC
- COLOPLAST GROUP
- ORGANOGENESIS INC.
- PAUL HARTMANN AG
- MIMEDX GROUP, INC.
- OWENS & MINOR
- B. BRAUN SE
- ESSITY AKTIEBOLAG
- INTEGRA LIFESCIENCES CORPORATION
- AVERY DENNISON CORPORATION
- MATIV HOLDINGS, INC.
- BIOVENTUS
- ZIMMER BIOMET
- MEDTRONIC
- BAXTER
- 其他公司
- LOHMANN & RAUSCHER GMBH & CO. KG
- MEDLINE INDUSTRIES, LP
- DEROYAL INDUSTRIES, INC.
- WINNER MEDICAL CO., LTD.
- ADVANCIS (UK)
- MIL LABORATORIES PVT. LTD.
- PENSAR MEDICAL
- HAROMED BV
- URGO GROUP
- DIRECT HEALTHCARE GROUP
第12章 附錄
The global wound care market is projected to reach USD 30.48 billion by 2030 from USD 22.22 billion in 2025, at a CAGR of 6.5% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Product, Wound type, and End User |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
This is due to the increasing rise of diabetic patients, rising cases of traumatic injuries and burn injuries, and the growing geriatric population, which drives market growth. Additionally, the risk of advanced wound care products restraining the market growth.

The hospitals and clinics segment of the wound care market, by end user, is expected to hold the largest position during the forecast period.
Based on end user, the market is segmented into hospitals and clinics, home care settings, long-term care facilities, and other end users. In 2024, hospitals and clinics account for the largest share of the market. This dominance is driven by the rising prevalence of diabetic foot ulcers and surgical wounds, which require professional medical attention. Additionally, the aging population with chronic conditions such as diabetes and vascular disorders has led to an increase in hospital visits for wound care. Hospitals and specialized care centers are well equipped with experienced medical personnel, standardized treatment protocols, and a comprehensive range of wound care products, enabling efficient and effective treatment. The continued emphasis on enhancing wound care outcomes and patient recovery further reinforces the leading position of this segment.
Asia Pacific is the fastest-growing region of the wound care market.
The global wound care market is segmented into five segments, namely, North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. The Asia Pacific wound care market is expanding due to a growing aging population and a rise in lifestyle-related diseases that contribute to chronic wounds. Increased demand for quality healthcare and greater investments in hospital infrastructure and medical technologies are boosting the adoption of advanced wound care products. Moreover, the surge in medical tourism across countries like India, Thailand, and Singapore is driving surgical volumes, further increasing the need for post-operative wound care. As healthcare providers in the region focus on improving treatment outcomes and reducing recovery times, the market is poised for robust growth, with Asia Pacific projected to witness the highest CAGR during the forecast period.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1: 40%, Tier 2: 30%, and Tier 3: 30%
- By Designation: C Level: 27%, Director Level: 18%, and Others: 55%
- By Region: North America: 51%, Europe: 21%, Asia Pacific: 18%, Latin America: 6%, and Middle East & Africa: 4%
Note 1: Companies are classified into tiers based on their total revenue. As of 2023, Tier 1 = >USD 10.00 billion, Tier 2 = USD 1.00 billion to USD 10.00 billion, and Tier 3 = <USD 1.00 billion.
Note 2: C-level primaries include CEOs, CFOs, COOs, and VPs.
Note 3: Other designations include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
The major players operating in the wound care market are Solventum (US), Johnson & Johnson Services, Inc. (US), Smith+ Nephew (UK), Convatec Group PLC (UK), Coloplast Group (Denmark), Cardinal Health (US), Molnlycke AB (Sweden), Integra LifeSciences Corporation (US), PAUL HARTMANN AG (Germany), B.Braun SE (Germany), Organogenesis Inc. (US), MIMEDX Group, Inc. (US), Essity Aktlebolag (Sweden), Avery Dennison Corporation (US), Mativ Holdings, Inc. (US), Owens & Minor (US), Zimmer Biomet (US), Bioventus (US), Medtronic (Ireland), and Baxter (US).
Research Coverage
This report studies the wound care market based on product, wound type, end user, and country. The report also studies factors (such as drivers, restraints, opportunities, and challenges) affecting market growth and provides details of the competitive landscape for market leaders. Furthermore, the report analyzes micro markets with respect to their individual growth trends. It forecasts the revenue of the market segments with respect to five major regions (and the respective countries in these regions).
Reasons to Buy the Report
The report will enable established firms as well as entrants/smaller firms to gauge the pulse of the market, which, in turn, would help them to garner a larger market share. Firms purchasing the report could use one or a combination of the strategies mentioned below to strengthen their market presence.
This report provides insights on the following pointers:
- Analysis of key drivers (growing geriatric population, increasing cases of traumatic injuries, growing prevalence of chronic diseases
rising incidence of burn injuries, rising use of regenerative medicine for wound management,
and government initiatives and reimbursement policies), restraints (high cost of advanced wound care products, risks associated with use of advanced wound care products
and antimicrobial resistance challenges), opportunities (Growth opportunities in emerging economies, technological advancement in wound care, and home-care optimized solutions), challenges (lack of trained healthcare professionals, Limited awareness in underdeveloped regions, and data security and privacy concerns)
- Market Penetration: Comprehensive information on the product portfolios offered by the top players in the wound care market
- Product Development/Innovation: Detailed insights on the upcoming trends, R&D activities, and product launches in the wound care market
- Market Development: Comprehensive information on lucrative emerging regions
- Market Diversification: Exhaustive information about new products, growing geographies, and recent developments in the wound care market
- Competitive Assessment: In-depth assessment of market segments, growth strategies, revenue analysis, and products of the leading market players
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION AND REGIONAL SCOPE
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.4 YEARS CONSIDERED
- 1.5 CURRENCY CONSIDERED
- 1.6 STAKEHOLDERS
- 1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Primary sources
- 2.1.2.2 Key industry insights
- 2.1.2.3 Key data from primary sources
- 2.1.2.4 Breakdown of primary interviews
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 BOTTOM-UP APPROACH
- 2.2.1.1 Approach 1: Company revenue estimation approach
- 2.2.1.2 Approach 2: Presentations of companies
- 2.2.1.3 Approach 3: Primary interviews
- 2.2.1.4 Growth forecast
- 2.2.1.5 CAGR projections
- 2.2.2 TOP-DOWN APPROACH
- 2.2.1 BOTTOM-UP APPROACH
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.4 RESEARCH ASSUMPTIONS
- 2.4.1 STUDY-RELATED ASSUMPTIONS
- 2.4.2 ASSUMPTIONS, BY PARAMETER
- 2.4.3 GROWTH RATE ASSUMPTIONS
- 2.5 RESEARCH LIMITATIONS
- 2.6 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 WOUND CARE MARKET OVERVIEW
- 4.2 ASIA PACIFIC: WOUND CARE MARKET SHARE, BY END USER AND COUNTRY
- 4.3 WOUND CARE MARKET, BY KEY COUNTRY
- 4.4 WOUND CARE MARKET: REGIONAL MIX, 2023-2030
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Growing geriatric population
- 5.2.1.2 Rise in cases of traumatic injuries
- 5.2.1.3 Growing prevalence of chronic diseases
- 5.2.1.4 Rising incidence of burn injuries
- 5.2.1.5 Rising use of regenerative medicines for wound management
- 5.2.1.6 Government initiatives and reimbursement policies
- 5.2.2 RESTRAINTS
- 5.2.2.1 High cost of advanced wound care products
- 5.2.2.2 Risks associated with advanced wound care products
- 5.2.2.3 Antimicrobial resistance challenges
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growth opportunities in emerging economies
- 5.2.3.2 Technological advancements in wound care
- 5.2.3.3 Home care-optimized solutions
- 5.2.4 CHALLENGES
- 5.2.4.1 Lack of trained healthcare professionals
- 5.2.4.2 Limited access to wound care solutions in underdeveloped regions
- 5.2.4.3 Data security and privacy concerns
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.4 PRICING ANALYSIS
- 5.4.1 AVERAGE SELLING PRICE TREND, BY PRODUCT
- 5.4.2 AVERAGE SELLING PRICE TREND OF WOUND CARE PRODUCTS FOR KEY WOUND TYPES, BY KEY PLAYER
- 5.4.3 AVERAGE SELLING PRICE TREND, BY REGION
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- 5.8 INVESTMENT & FUNDING SCENARIO
- 5.9 TECHNOLOGY ANALYSIS
- 5.9.1 KEY TECHNOLOGIES
- 5.9.1.1 Negative pressure wound therapy
- 5.9.2 COMPLEMENTARY TECHNOLOGIES
- 5.9.2.1 Micropore particle technology
- 5.9.3 ADJACENT TECHNOLOGIES
- 5.9.3.1 Telemedicine and Mobile Health (MHealth)
- 5.9.1 KEY TECHNOLOGIES
- 5.10 PATENT ANALYSIS
- 5.11 TRADE ANALYSIS
- 5.11.1 IMPORT SCENARIO
- 5.11.2 EXPORT SCENARIO
- 5.12 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.13 CASE STUDY ANALYSIS
- 5.13.1 CASE STUDY 1: ADOPTION OF ADVANCED WOUND CARE MEASURES FOR COMPLEX WOUND HEALING
- 5.13.2 CASE STUDY 2: EVALUATION OF NEW HYDROCOLL HYDROCOLLOID DRESSING IN WOUND CARE
- 5.13.3 CASE STUDY 3: SUPPORTED SELF-MANAGEMENT OF HARD-TO-HEAL PRESSURE ULCERS USING SIMPLE 3-STEP FRAMEWORK DEVELOPED BY COLOPLAST
- 5.14 REGULATORY LANDSCAPE
- 5.14.1 REGULATORY ANALYSIS
- 5.14.1.1 North America
- 5.14.1.1.1 US
- 5.14.1.1.2 Canada
- 5.14.1.2 Europe
- 5.14.1.2.1 Germany
- 5.14.1.2.2 UK
- 5.14.1.2.3 France
- 5.14.1.3 Asia Pacific
- 5.14.1.3.1 China
- 5.14.1.3.2 Japan
- 5.14.1.3.3 India
- 5.14.1.4 Latin America
- 5.14.1.5 Middle East
- 5.14.1.1 North America
- 5.14.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.14.2.1 North America
- 5.14.2.2 Europe
- 5.14.2.3 Asia Pacific
- 5.14.2.4 Latin America
- 5.14.2.5 Rest of the World
- 5.14.1 REGULATORY ANALYSIS
- 5.15 PORTER'S FIVE FORCES ANALYSIS
- 5.15.1 BARGAINING POWER OF SUPPLIERS
- 5.15.2 BARGAINING POWER OF BUYERS
- 5.15.3 THREAT FROM NEW ENTRANTS
- 5.15.4 THREAT FROM SUBSTITUTES
- 5.15.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.16 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.16.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.16.2 BUYING CRITERIA
- 5.17 IMPACT OF AI ON WOUND CARE MARKET
- 5.17.1 INTRODUCTION
- 5.17.2 MARKET POTENTIAL OF AI IN WOUND CARE MARKET
- 5.17.3 IMPACT OF AI ON WOUND CARE MARKET: USE CASES
- 5.17.4 KEY COMPANIES IMPLEMENTING AI
- 5.17.5 FUTURE OF AI IN WOUND CARE MARKET
- 5.18 US 2025 TARIFF
- 5.18.1 INTRODUCTION
- 5.18.2 KEY TARIFF RATES
- 5.18.3 PRICE IMPACT ANALYSIS
- 5.18.4 IMPACT ON COUNTRY/REGION
- 5.18.4.1 North America
- 5.18.4.2 Europe
- 5.18.4.3 Asia Pacific
- 5.18.5 IMPACT ON END-USE INDUSTRIES
- 5.18.5.1 Hospitals and clinics
6 WOUND CARE MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- 6.2 ADVANCED WOUND CARE PRODUCTS
- 6.2.1 ADVANCED WOUND DRESSINGS, BY TYPE
- 6.2.1.1 Foam dressings
- 6.2.1.1.1 Silicone dressings
- 6.2.1.1.1.1 Ability of silicone dressings to expedite wound closure and reduce risk of maceration to drive market
- 6.2.1.1.2 Non-silicone dressings
- 6.2.1.1.2.1 Non-silicone dressings are designed for patient comfort and ease of use
- 6.2.1.1.1 Silicone dressings
- 6.2.1.2 Hydrocolloid dressings
- 6.2.1.2.1 Ability of hydrocolloid dressings to promote granulation and formation of new tissues in open wounds to boost demand
- 6.2.1.3 Film dressings
- 6.2.1.3.1 Film dressings offer strong adhesive properties and adaptability to various wound types
- 6.2.1.4 Alginate dressings
- 6.2.1.4.1 Growing incidence of pressure and diabetic foot ulcers to fuel growth
- 6.2.1.5 Hydrogel dressings
- 6.2.1.5.1 Ability of hydrogel dressings to provide relief and cooling effect on skin to drive market
- 6.2.1.6 Collagen dressings
- 6.2.1.6.1 Favorable reimbursement scenario for collagen dressings to support market
- 6.2.1.7 Hydrofiber dressings
- 6.2.1.7.1 Combination of properties of hydrocolloids and alginates to boost adoption of hydrofiber dressings
- 6.2.1.8 Wound contact layers
- 6.2.1.8.1 Ability of wound contact layers to protect wound beds from bacterial and fungal growth to drive market
- 6.2.1.9 Superabsorbent dressings
- 6.2.1.9.1 Use of superabsorbent dressings for fragile skin to promote growth
- 6.2.1.10 Other advanced wound care dressings
- 6.2.1.1 Foam dressings
- 6.2.2 ADVANCED WOUND DRESSINGS, BY PROPERTY
- 6.2.2.1 Non-antimicrobial dressings
- 6.2.2.1.1 Need for cost-effective dressing alternatives to drive market
- 6.2.2.2 Antimicrobial dressings
- 6.2.2.2.1 Advanced antimicrobial dressings are designed to prevent or manage infections in wounds
- 6.2.2.1 Non-antimicrobial dressings
- 6.2.3 DEVICES & ACCESSORIES
- 6.2.3.1 NPWT devices & accessories
- 6.2.3.1.1 Growing adoption of single-use NPWT devices in home care to drive growth
- 6.2.3.2 Debridement devices & accessories
- 6.2.3.2.1 Focus on developing novel technologies to support market
- 6.2.3.3 Wound assessment & monitoring devices
- 6.2.3.3.1 Potential cost-reducing capabilities of wound assessment & monitoring devices to boost adoption
- 6.2.3.4 Other devices & accessories
- 6.2.3.1 NPWT devices & accessories
- 6.2.4 BIOLOGICAL SKIN SUBSTITUTES
- 6.2.4.1 Human donor tissue-derived products
- 6.2.4.1.1 High efficacy of human donor tissue-derived products to support market demand
- 6.2.4.2 Acellular animal-derived products
- 6.2.4.2.1 Recognition of importance of ECM in wound treatment to lead to development of acellular wound care biologics
- 6.2.4.3 Biosynthetic products
- 6.2.4.3.1 Increased bioburden of infected wounds to lead to development of biosynthetic wound care products
- 6.2.4.1 Human donor tissue-derived products
- 6.2.5 TOPICAL AGENTS
- 6.2.5.1 Cost-effectiveness and ease of use of topical agents in home care settings to drive demand
- 6.2.1 ADVANCED WOUND DRESSINGS, BY TYPE
- 6.3 SURGICAL WOUND CARE PRODUCTS
- 6.3.1 SUTURES
- 6.3.1.1 High tensile strength of sutures to drive market
- 6.3.2 TISSUE ADHESIVES, SEALANTS, AND GLUES
- 6.3.2.1 Fibrin-based sealants
- 6.3.2.1.1 Fibrin-based sealants replicate final stages of body's natural blood-clotting process
- 6.3.2.2 Synthetic adhesives
- 6.3.2.2.1 Synthetic adhesives have additional sealing properties than traditional staples
- 6.3.2.3 Collagen-based sealants
- 6.3.2.3.1 Collagen-based sealants are primarily used in surgical procedures to support hemostasis
- 6.3.2.1 Fibrin-based sealants
- 6.3.3 STAPLERS
- 6.3.3.1 Staple lines are consistent and less likely to leak blood or air
- 6.3.1 SUTURES
- 6.4 TRADITIONAL WOUND CARE PRODUCTS
- 6.4.1 FIXATIONS
- 6.4.1.1 Fixation materials ensure dressings, wound pads, and compresses stay securely in place
- 6.4.2 BANDAGES
- 6.4.2.1 Wide usage of bandages to support market growth
- 6.4.3 GAUZES
- 6.4.3.1 Wide usage of gauzes in surgeries and wound packing to propel market growth
- 6.4.4 FIRST AID PLASTERS
- 6.4.4.1 Need for rapid wound management and protection to propel adoption
- 6.4.5 ABSORBENT PADS
- 6.4.5.1 Wide usage of absorbent pads in wound dressing and exudate management to drive market
- 6.4.6 COMPRESSIONS
- 6.4.6.1 Ability of compression products to reduce swelling to boost demand
- 6.4.7 ISLAND DRESSINGS
- 6.4.7.1 Waterproof, antimicrobial, and absorbent properties of island dressings to drive their demand
- 6.4.8 OTHER TRADITIONAL WOUND CARE PRODUCTS
- 6.4.1 FIXATIONS
7 WOUND CARE MARKET, BY WOUND TYPE
- 7.1 INTRODUCTION
- 7.2 CHRONIC WOUNDS
- 7.2.1 DIABETIC FOOT ULCERS
- 7.2.1.1 Rising incidence of diabetes to drive market growth
- 7.2.2 PRESSURE ULCERS
- 7.2.2.1 Growing geriatric population to drive market growth
- 7.2.3 VENOUS LEG ULCERS
- 7.2.3.1 Rising prevalence of obesity to drive market growth
- 7.2.4 OTHER CHRONIC WOUNDS
- 7.2.1 DIABETIC FOOT ULCERS
- 7.3 ACUTE WOUNDS
- 7.3.1 SURGICAL & TRAUMATIC WOUNDS
- 7.3.1.1 Rising volume of surgical procedures to drive market
- 7.3.2 BURNS
- 7.3.2.1 High incidence of burn injuries in developing regions to support market growth
- 7.3.1 SURGICAL & TRAUMATIC WOUNDS
8 WOUND CARE MARKET, BY END USER
- 8.1 INTRODUCTION
- 8.2 HOSPITALS & CLINICS
- 8.2.1 INPATIENT SETTINGS
- 8.2.1.1 Increasing hospital admissions due to HAIs to drive market
- 8.2.2 OUTPATIENT SETTINGS
- 8.2.2.1 Rising cost of inpatient facilities to spur market for outpatient care settings
- 8.2.1 INPATIENT SETTINGS
- 8.3 HOME CARE SETTINGS
- 8.3.1 DECREASED COSTS AND HIGH CONVENIENCE AND COMFORT OF HOME CARE SETTINGS TO ENSURE MARKET GROWTH
- 8.4 LONG-TERM CARE FACILITIES
- 8.4.1 GROWING GERIATRIC POPULATION TO BOOST NEED FOR BETTER LONG-TERM CARE
- 8.5 OTHER END USERS
9 WOUND CARE MARKET, BY REGION
- 9.1 INTRODUCTION
- 9.2 NORTH AMERICA
- 9.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 9.2.2 US
- 9.2.2.1 Rising prevalence of diabetes and supportive reimbursement policies to drive growth
- 9.2.3 CANADA
- 9.2.3.1 Government-backed efforts to support market development
- 9.3 EUROPE
- 9.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 9.3.2 GERMANY
- 9.3.2.1 Rising cases of diabetes and chronic wound burden to boost market
- 9.3.3 FRANCE
- 9.3.3.1 Rising chronic wound cases to drive market expansion
- 9.3.4 UK
- 9.3.4.1 Increase in R&D activity to propel market growth
- 9.3.5 ITALY
- 9.3.5.1 Increased healthcare funding and sustained demand for treatment solutions to drive market
- 9.3.6 SPAIN
- 9.3.6.1 Rising life expectancy and aging population to boost adoption of wound care products
- 9.3.7 RUSSIA
- 9.3.7.1 Government initiatives to raise awareness about diabetes to support market expansion
- 9.3.8 REST OF EUROPE
- 9.4 ASIA PACIFIC
- 9.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 9.4.2 CHINA
- 9.4.2.1 Increasing incidence of diabetic foot ulcers (DFUs) to encourage growth
- 9.4.3 JAPAN
- 9.4.3.1 Evolving healthcare delivery and infrastructure landscape to fuel market
- 9.4.4 INDIA
- 9.4.4.1 Booming medical tourism to speed up growth
- 9.4.5 AUSTRALIA
- 9.4.5.1 Growth of medical manufacturing companies to drive market
- 9.4.6 SOUTH KOREA
- 9.4.6.1 Increasing healthcare spending to sustain growth
- 9.4.7 SINGAPORE
- 9.4.7.1 Increasing focus on wound care therapies to facilitate growth
- 9.4.8 REST OF ASIA PACIFIC
- 9.5 LATIN AMERICA
- 9.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 9.5.2 BRAZIL
- 9.5.2.1 Growth in healthcare sector to drive market
- 9.5.3 MEXICO
- 9.5.3.1 Expanding hospital infrastructure to aid growth
- 9.5.4 REST OF LATIN AMERICA
- 9.6 MIDDLE EAST & AFRICA
- 9.6.1 GROWING DEMAND FOR HEALTHCARE FACILITIES TO DRIVE MARKET
- 9.6.2 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
10 COMPETITIVE LANDSCAPE
- 10.1 OVERVIEW
- 10.2 KEY PLAYER STRATEGIES/RIGHT TO WIN, 2024
- 10.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN WOUND CARE MARKET
- 10.3 REVENUE ANALYSIS, 2022-2024
- 10.4 MARKET SHARE ANALYSIS, 2024
- 10.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 10.5.1 STARS
- 10.5.2 EMERGING LEADERS
- 10.5.3 PERVASIVE PLAYERS
- 10.5.4 PARTICIPANTS
- 10.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 10.5.5.1 Company footprint
- 10.5.5.2 Region footprint
- 10.5.5.3 Product footprint
- 10.5.5.4 Wound type footprint
- 10.5.5.5 End user footprint
- 10.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 10.6.1 PROGRESSIVE COMPANIES
- 10.6.2 RESPONSIVE COMPANIES
- 10.6.3 DYNAMIC COMPANIES
- 10.6.4 STARTING BLOCKS
- 10.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 10.6.5.1 Detailed list of key startups/SMEs
- 10.6.5.2 Competitive benchmarking of key startups/SMEs
- 10.7 COMPANY VALUATION AND FINANCIAL METRICS
- 10.7.1 COMPANY VALUATION
- 10.7.2 FINANCIAL METRICS
- 10.8 BRAND/PRODUCT COMPARISON
- 10.9 COMPETITIVE SCENARIO
- 10.9.1 PRODUCT LAUNCHES & APPROVALS
- 10.9.2 DEALS
- 10.9.3 EXPANSIONS
- 10.9.4 OTHER DEVELOPMENTS
11 COMPANY PROFILES
- 11.1 KEY PLAYERS
- 11.1.1 SOLVENTUM
- 11.1.1.1 Business overview
- 11.1.1.2 Products offered
- 11.1.1.3 Recent developments
- 11.1.1.3.1 Product launches & approvals
- 11.1.1.3.2 Other developments
- 11.1.1.4 MnM view
- 11.1.1.4.1 Right to win
- 11.1.1.4.2 Strategic choices
- 11.1.1.4.3 Weaknesses & competitive threats
- 11.1.2 JOHNSON & JOHNSON SERVICES, INC. (ETHICON)
- 11.1.2.1 Business overview
- 11.1.2.2 Products offered
- 11.1.2.3 Recent developments
- 11.1.2.3.1 Product launches & approvals
- 11.1.2.4 MnM view
- 11.1.2.4.1 Right to win
- 11.1.2.4.2 Strategic choices
- 11.1.2.4.3 Weaknesses & competitive threats
- 11.1.3 SMITH+NEPHEW
- 11.1.3.1 Business overview
- 11.1.3.2 Products offered
- 11.1.3.3 Recent developments
- 11.1.3.3.1 Product launches & approvals
- 11.1.3.3.2 Deals
- 11.1.3.3.3 Expansions
- 11.1.3.3.4 Other developments
- 11.1.3.4 MnM view
- 11.1.3.4.1 Right to win
- 11.1.3.4.2 Strategic choices
- 11.1.3.4.3 Weaknesses & competitive threats
- 11.1.4 CARDINAL HEALTH
- 11.1.4.1 Business overview
- 11.1.4.2 Products offered
- 11.1.4.3 Recent developments
- 11.1.4.3.1 Deals
- 11.1.4.3.2 Expansions
- 11.1.4.4 MnM view
- 11.1.4.4.1 Right to win
- 11.1.4.4.2 Strategic choices
- 11.1.4.4.3 Weaknesses & competitive threats
- 11.1.5 MOLNLYCKE AB
- 11.1.5.1 Business overview
- 11.1.5.2 Products offered
- 11.1.5.3 Recent developments
- 11.1.5.3.1 Deals
- 11.1.5.3.2 Other developments
- 11.1.5.4 MnM view
- 11.1.5.4.1 Right to win
- 11.1.5.4.2 Strategic choices
- 11.1.5.4.3 Weaknesses & competitive threats
- 11.1.6 CONVATEC GROUP PLC
- 11.1.6.1 Business overview
- 11.1.6.2 Products offered
- 11.1.6.3 Recent developments
- 11.1.6.3.1 Product launches & approvals
- 11.1.6.3.2 Deals
- 11.1.7 COLOPLAST GROUP
- 11.1.7.1 Business overview
- 11.1.7.2 Products offered
- 11.1.7.3 Recent developments
- 11.1.7.3.1 Product launches & approvals
- 11.1.7.3.2 Deals
- 11.1.8 ORGANOGENESIS INC.
- 11.1.8.1 Business overview
- 11.1.8.2 Products offered
- 11.1.8.3 Recent developments
- 11.1.8.3.1 Product launches & approvals
- 11.1.8.3.2 Expansions
- 11.1.9 PAUL HARTMANN AG
- 11.1.9.1 Business overview
- 11.1.9.2 Products offered
- 11.1.9.3 Recent developments
- 11.1.9.3.1 Deals
- 11.1.10 MIMEDX GROUP, INC.
- 11.1.10.1 Business overview
- 11.1.10.2 Products offered
- 11.1.10.3 Recent developments
- 11.1.10.3.1 Product launches & approvals
- 11.1.11 OWENS & MINOR
- 11.1.11.1 Business overview
- 11.1.11.2 Products offered
- 11.1.11.3 Recent developments
- 11.1.11.3.1 Deals
- 11.1.11.3.2 Other developments
- 11.1.12 B. BRAUN SE
- 11.1.12.1 Business overview
- 11.1.12.2 Products offered
- 11.1.12.3 Recent developments
- 11.1.12.3.1 Expansions
- 11.1.12.3.2 Other developments
- 11.1.13 ESSITY AKTIEBOLAG
- 11.1.13.1 Business overview
- 11.1.13.2 Products offered
- 11.1.13.3 Recent developments
- 11.1.13.3.1 Other developments
- 11.1.14 INTEGRA LIFESCIENCES CORPORATION
- 11.1.14.1 Business overview
- 11.1.14.2 Products offered
- 11.1.14.3 Recent developments
- 11.1.14.3.1 Product launches & approvals
- 11.1.14.3.2 Other developments
- 11.1.15 AVERY DENNISON CORPORATION
- 11.1.15.1 Business overview
- 11.1.15.2 Products offered
- 11.1.15.3 Recent developments
- 11.1.15.3.1 Expansions
- 11.1.16 MATIV HOLDINGS, INC.
- 11.1.16.1 Business overview
- 11.1.16.2 Products offered
- 11.1.16.3 Recent developments
- 11.1.16.3.1 Deals
- 11.1.17 BIOVENTUS
- 11.1.17.1 Business overview
- 11.1.17.2 Products offered
- 11.1.17.3 Recent developments
- 11.1.17.3.1 Deals
- 11.1.17.3.2 Other developments
- 11.1.18 ZIMMER BIOMET
- 11.1.18.1 Business overview
- 11.1.18.2 Products offered
- 11.1.18.3 Recent developments
- 11.1.18.3.1 Deals
- 11.1.19 MEDTRONIC
- 11.1.19.1 Business overview
- 11.1.19.2 Products offered
- 11.1.20 BAXTER
- 11.1.20.1 Business overview
- 11.1.20.2 Products offered
- 11.1.20.3 Recent developments
- 11.1.20.3.1 Product launches & approvals
- 11.1.1 SOLVENTUM
- 11.2 OTHER PLAYERS
- 11.2.1 LOHMANN & RAUSCHER GMBH & CO. KG
- 11.2.2 MEDLINE INDUSTRIES, LP
- 11.2.3 DEROYAL INDUSTRIES, INC.
- 11.2.4 WINNER MEDICAL CO., LTD.
- 11.2.5 ADVANCIS (UK)
- 11.2.6 MIL LABORATORIES PVT. LTD.
- 11.2.7 PENSAR MEDICAL
- 11.2.8 HAROMED BV
- 11.2.9 URGO GROUP
- 11.2.10 DIRECT HEALTHCARE GROUP
12 APPENDIX
- 12.1 DISCUSSION GUIDE
- 12.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 12.3 CUSTOMIZATION OPTIONS
- 12.4 RELATED REPORTS
- 12.5 AUTHOR DETAILS
List of Tables
- TABLE 1 WOUND CARE MARKET: INCLUSIONS AND EXCLUSIONS
- TABLE 2 WOUND CARE MARKET: RISK ASSESSMENT
- TABLE 3 POPULATION SHARE OF PEOPLE AGED 65 AND ABOVE (% OF TOTAL POPULATION), BY COUNTRY, 2023
- TABLE 4 ROAD ACCIDENT DEMOGRAPHICS, 2022
- TABLE 5 CHINA: STATE OF PATIENTS (WITH TYPE 2 DFU) ON DISCHARGE, BY AGE GROUP, JANUARY 2017-DECEMBER 2023
- TABLE 6 RISKS ASSOCIATED WITH ADVANCED WOUND CARE DEVICES
- TABLE 7 STRATEGIC DEVELOPMENTS IN ASIA PACIFIC, 2023-2024
- TABLE 8 AVERAGE SELLING PRICING TREND OF WOUND CARE PRODUCTS, 2023-2025
- TABLE 9 AVERAGE SELLING PRICE TREND OF WOUND CARE PRODUCTS FOR KEY WOUND TYPES, BY KEY PLAYER, 2023-2025
- TABLE 10 AVERAGE SELLING PRICE TREND OF WOUND CARE PRODUCTS, BY REGION, 2023-2025
- TABLE 11 WOUND CARE MARKET: ROLE OF PLAYERS IN ECOSYSTEM
- TABLE 12 WOUND CARE MARKET: INNOVATIONS AND PATENT REGISTRATIONS, 2023-2025
- TABLE 13 IMPORT DATA FOR HS CODE 300510-COMPLIANT PRODUCTS, BY COUNTRY, 2020-2024 (USD MILLION)
- TABLE 14 EXPORT DATA FOR HS CODE 300510-COMPLIANT PRODUCTS, BY COUNTRY, 2020-2024 (USD MILLION)
- TABLE 15 WOUND CARE MARKET: KEY CONFERENCES AND EVENTS, APRIL 2025-DECEMBER 2026
- TABLE 16 TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS IN JAPAN
- TABLE 17 NORTH AMERICA: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 18 EUROPE: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 19 ASIA PACIFIC: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 20 LATIN AMERICA: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 21 REST OF THE WORLD: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 22 IMPACT OF PORTER'S FORCES ON WOUND CARE MARKET
- TABLE 23 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY KEY PRODUCT (%)
- TABLE 24 KEY BUYING CRITERIA FOR KEY PRODUCTS
- TABLE 25 RECIPROCAL TARIFF RATES ADJUSTED BY US
- TABLE 26 WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 27 ADVANCED WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 28 ADVANCED WOUND CARE PRODUCTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 29 NORTH AMERICA: ADVANCED WOUND CARE PRODUCTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 30 EUROPE: ADVANCED WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 31 ASIA PACIFIC: ADVANCED WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 32 LATIN AMERICA: ADVANCED WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 33 ADVANCED WOUND DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 34 ADVANCED WOUND DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 35 NORTH AMERICA: ADVANCED WOUND DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 36 EUROPE: ADVANCED WOUND DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 37 ASIA PACIFIC: ADVANCED WOUND DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 38 LATIN AMERICA: WOUND CARE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 39 KEY PRODUCT TYPES IN FOAM DRESSINGS MARKET
- TABLE 40 FOAM DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 41 FOAM DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 42 NORTH AMERICA: FOAM DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 43 EUROPE: FOAM DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 44 ASIA PACIFIC: FOAM DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 45 LATIN AMERICA: FOAM DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 46 SILICONE DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 47 NORTH AMERICA: SILICONE DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 48 EUROPE: SILICONE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 49 ASIA PACIFIC: SILICONE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 50 LATIN AMERICA: SILICONE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 51 NON-SILICONE DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 52 NORTH AMERICA: NON-SILICONE DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 53 EUROPE: NON-SILICONE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 54 ASIA PACIFIC: NON-SILICONE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 55 LATIN AMERICA: NON-SILICONE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 56 KEY PRODUCT TYPES IN HYDROCOLLOID DRESSINGS MARKET, BY COMPANY
- TABLE 57 HYDROCOLLOID DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 58 NORTH AMERICA: HYDROCOLLOID DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 59 EUROPE: HYDROCOLLOID DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 60 ASIA PACIFIC: HYDROCOLLOID DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 61 LATIN AMERICA: HYDROCOLLOID DRESSINGS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 62 KEY PRODUCT TYPES IN FILM DRESSINGS MARKET, BY COMPANY
- TABLE 63 FILM DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 64 NORTH AMERICA: FILM DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 65 EUROPE: FILM DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 66 ASIA PACIFIC: FILM DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 67 LATIN AMERICA: FILM DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 68 KEY PRODUCT TYPES IN ALGINATE DRESSINGS MARKET, BY COMPANY
- TABLE 69 ALGINATE DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 70 NORTH AMERICA: ALGINATE DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 71 EUROPE: ALGINATE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 72 ASIA PACIFIC: ALGINATE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 73 LATIN AMERICA: ALGINATE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 74 KEY PRODUCT TYPES IN HYDROGEL DRESSINGS MARKET, BY COMPANY
- TABLE 75 HYDROGEL DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 76 NORTH AMERICA: HYDROGEL DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 77 EUROPE: HYDROGEL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 78 ASIA PACIFIC: HYDROGEL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 79 LATIN AMERICA: HYDROGEL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 80 KEY PRODUCT TYPES IN COLLAGEN DRESSINGS MARKET, BY COMPANY
- TABLE 81 COLLAGEN DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 82 NORTH AMERICA: COLLAGEN DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 83 EUROPE: COLLAGEN DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 84 ASIA PACIFIC: COLLAGEN DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 85 LATIN AMERICA: COLLAGEN DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 86 KEY PRODUCT TYPES IN HYDROFIBER DRESSINGS MARKET, BY COMPANY
- TABLE 87 HYDROFIBER DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 88 NORTH AMERICA: HYDROFIBER DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 89 EUROPE: HYDROFIBER DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 90 ASIA PACIFIC: HYDROFIBER DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 91 LATIN AMERICA: HYDROFIBER DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 92 KEY PRODUCT TYPES IN WOUND CONTACT LAYERS MARKET, BY COMPANY
- TABLE 93 WOUND CONTACT LAYERS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 94 NORTH AMERICA: WOUND CONTACT LAYERS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 95 EUROPE: WOUND CONTACT LAYERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 96 ASIA PACIFIC: WOUND CONTACT LAYERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 97 LATIN AMERICA: WOUND CONTACT LAYERS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 98 KEY PRODUCT TYPES IN SUPERABSORBENT DRESSINGS MARKET, BY COMPANY
- TABLE 99 SUPERABSORBENT DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 100 NORTH AMERICA: SUPERABSORBENT DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 101 EUROPE: SUPERABSORBENT DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 102 ASIA PACIFIC: SUPERABSORBENT DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 103 LATIN AMERICA: SUPERABSORBENT DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 104 KEY PRODUCT TYPES IN OTHER ADVANCED WOUND CARE DRESSINGS MARKET, BY COMPANY
- TABLE 105 OTHER ADVANCED WOUND CARE DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 106 NORTH AMERICA: OTHER ADVANCED WOUND CARE DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 107 EUROPE: OTHER ADVANCED WOUND CARE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 108 ASIA PACIFIC: OTHER ADVANCED WOUND CARE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 109 LATIN AMERICA: OTHER ADVANCED WOUND CARE DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 110 ADVANCED WOUND DRESSINGS MARKET, BY PROPERTY, 2023-2030 (USD MILLION)
- TABLE 111 NON-ANTIMICROBIAL DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 112 NORTH AMERICA: NON-ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 113 EUROPE: NON-ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 114 ASIA PACIFIC: NON-ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 115 LATIN AMERICA: NON-ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 116 ANTIMICROBIAL DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 117 NORTH AMERICA: ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 118 EUROPE: ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 119 ASIA PACIFIC: ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 120 LATIN AMERICA: ANTIMICROBIAL DRESSINGS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 121 DEVICES & ACCESSORIES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 122 DEVICES & ACCESSORIES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 123 NORTH AMERICA: DEVICES & ACCESSORIES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 124 EUROPE: DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 125 ASIA PACIFIC: DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 126 LATIN AMERICA: DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 127 KEY PRODUCT TYPES IN NPWT DEVICES & ACCESSORIES MARKET, BY COMPANY
- TABLE 128 NPWT DEVICES & ACCESSORIES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 129 NORTH AMERICA: NPWT DEVICES & ACCESSORIES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 130 EUROPE: NPWT DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 131 ASIA PACIFIC: NPWT DEVICES & ACCESSORIES MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 132 LATIN AMERICA: NPWT DEVICES & ACCESSORIES MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 133 KEY PRODUCT TYPES IN DEBRIDEMENT DEVICES & ACCESSORIES MARKET, BY COMPANY
- TABLE 134 DEBRIDEMENT DEVICES & ACCESSORIES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 135 NORTH AMERICA: DEBRIDEMENT DEVICES & ACCESSORIES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 136 EUROPE: DEBRIDEMENT DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 137 ASIA PACIFIC: DEBRIDEMENT DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 138 LATIN AMERICA: DEBRIDEMENT DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 139 KEY PRODUCT TYPES IN WOUND ASSESSMENT & MONITORING DEVICES MARKET, BY COMPANY
- TABLE 140 WOUND ASSESSMENT & MONITORING DEVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 141 NORTH AMERICA: WOUND ASSESSMENT & MONITORING DEVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 142 EUROPE: WOUND ASSESSMENT & MONITORING DEVICES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 143 ASIA PACIFIC: WOUND ASSESSMENT & MONITORING DEVICES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 144 LATIN AMERICA: WOUND ASSESSMENT & MONITORING DEVICES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 145 OTHER DEVICES & ACCESSORIES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 146 NORTH AMERICA: OTHER DEVICES & ACCESSORIES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 147 EUROPE: OTHER DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 148 ASIA PACIFIC: OTHER DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 149 LATIN AMERICA: OTHER DEVICES & ACCESSORIES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 150 KEY PRODUCT TYPES IN BIOLOGICAL SKIN SUBSTITUTES MARKET, BY COMPANY
- TABLE 151 BIOLOGICAL SKIN SUBSTITUTES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 152 BIOLOGICAL SKIN SUBSTITUTES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 153 NORTH AMERICA: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 154 EUROPE: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 155 ASIA PACIFIC: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 156 LATIN AMERICA: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 157 KEY PRODUCT TYPES IN HUMAN DONOR TISSUE-DERIVED MARKET, BY COMPANY
- TABLE 158 HUMAN DONOR TISSUE-DERIVED MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 159 NORTH AMERICA: HUMAN DONOR TISSUE-DERIVED MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 160 EUROPE: HUMAN DONOR TISSUE-DERIVED MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 161 ASIA PACIFIC: HUMAN DONOR TISSUE-DERIVED MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 162 LATIN AMERICA: HUMAN DONOR TISSUE-DERIVED MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 163 KEY PRODUCT TYPES IN ACELLULAR ANIMAL-DERIVED MARKET, BY COMPANY
- TABLE 164 ACELLULAR ANIMAL-DERIVED MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 165 NORTH AMERICA: ACELLULAR ANIMAL-DERIVED MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 166 EUROPE: ACELLULAR ANIMAL-DERIVED MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 167 ASIA PACIFIC: ACELLULAR ANIMAL-DERIVED MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 168 LATIN AMERICA: ACELLULAR ANIMAL-DERIVED MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 169 KEY PRODUCT TYPES IN BIOSYNTHETIC MARKET, BY COMPANY
- TABLE 170 BIOSYNTHETIC PRODUCTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 171 NORTH AMERICA: BIOSYNTHETIC PRODUCTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 172 EUROPE: BIOSYNTHETIC PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 173 ASIA PACIFIC: BIOSYNTHETIC PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 174 LATIN AMERICA: BIOSYNTHETIC PRODUCTS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 175 KEY PRODUCT TYPES IN TOPICAL AGENTS MARKET, BY COMPANY
- TABLE 176 TOPICAL AGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 177 NORTH AMERICA: TOPICAL AGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 178 EUROPE: TOPICAL AGENTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 179 ASIA PACIFIC: TOPICAL AGENTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 180 LATIN AMERICA: TOPICAL AGENTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 181 SURGICAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 182 SURGICAL WOUND CARE PRODUCTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 183 NORTH AMERICA: SURGICAL WOUND CARE PRODUCTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 184 EUROPE: SURGICAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 185 ASIA PACIFIC: SURGICAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 186 LATIN AMERICA: SURGICAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 187 KEY PRODUCT TYPES IN SUTURES MARKET, BY COMPANY
- TABLE 188 SUTURES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 189 NORTH AMERICA: SUTURES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 190 EUROPE: SUTURES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 191 ASIA PACIFIC: SUTURES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 192 LATIN AMERICA: SUTURES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 193 KEY PRODUCT TYPES IN TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY COMPANY
- TABLE 194 TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 195 TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 196 NORTH AMERICA: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 197 EUROPE: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 198 ASIA PACIFIC: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 199 LATIN AMERICA: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 200 FIBRIN-BASED SEALANTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 201 NORTH AMERICA: FIBRIN-BASED SEALANTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 202 EUROPE: FIBRIN-BASED SEALANTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 203 ASIA PACIFIC: FIBRIN-BASED SEALANTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 204 LATIN AMERICA: FIBRIN-BASED SEALANTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 205 SYNTHETIC ADHESIVES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 206 NORTH AMERICA: SYNTHETIC ADHESIVES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 207 EUROPE: SYNTHETIC ADHESIVES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 208 ASIA PACIFIC: SYNTHETIC ADHESIVES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 209 LATIN AMERICA: SYNTHETIC ADHESIVES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 210 COLLAGEN-BASED SEALANTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 211 NORTH AMERICA: COLLAGEN-BASED SEALANTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 212 EUROPE: COLLAGEN-BASED SEALANTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 213 ASIA PACIFIC: COLLAGEN-BASED SEALANTS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 214 LATIN AMERICA: COLLAGEN-BASED SEALANTS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 215 KEY PRODUCT TYPES IN STAPLERS MARKET, BY COMPANY
- TABLE 216 STAPLERS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 217 NORTH AMERICA: STAPLERS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 218 EUROPE: STAPLERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 219 ASIA PACIFIC: STAPLERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 220 LATIN AMERICA: STAPLERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 221 TRADITIONAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 222 TRADITIONAL WOUND CARE PRODUCTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 223 NORTH AMERICA: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 224 EUROPE: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 225 ASIA PACIFIC: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 226 LATIN AMERICA: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 227 KEY FIXATIONS OFFERED, BY COMPANY
- TABLE 228 FIXATIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 229 NORTH AMERICA: FIXATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 230 EUROPE: FIXATIONS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 231 ASIA PACIFIC: FIXATIONS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 232 LATIN AMERICA: FIXATIONS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 233 KEY BANDAGES OFFERED, BY COMPANY
- TABLE 234 BANDAGES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 235 NORTH AMERICA: BANDAGES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 236 EUROPE: BANDAGES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 237 ASIA PACIFIC: BANDAGES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 238 LATIN AMERICA: BANDAGES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 239 GAUZES OFFERED, BY COMPANY
- TABLE 240 GAUZES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 241 NORTH AMERICA: GAUZES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 242 EUROPE: GAUZES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 243 ASIA PACIFIC: GAUZES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 244 LATIN AMERICA: GAUZES MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 245 KEY FIRST AID PLASTER PRODUCTS OFFERED, BY COMPANY
- TABLE 246 FIRST AID PLASTERS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 247 NORTH AMERICA: FIRST AID PLASTERS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 248 EUROPE: FIRST AID PLASTERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 249 ASIA PACIFIC: FIRST AID PLASTERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 250 LATIN AMERICA: FIRST AID PLASTERS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 251 KEY ABSORBENT PADS OFFERED, BY COMPANY
- TABLE 252 ABSORBENT PADS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 253 NORTH AMERICA: ABSORBENT PADS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 254 EUROPE: ABSORBENT PADS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 255 ASIA PACIFIC: ABSORBENT PADS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 256 LATIN AMERICA: ABSORBENT PADS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 257 KEY COMPRESSIONS OFFERED, BY COMPANY
- TABLE 258 COMPRESSIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 259 NORTH AMERICA: COMPRESSIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 260 EUROPE: COMPRESSIONS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 261 ASIA PACIFIC: COMPRESSIONS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 262 LATIN AMERICA: COMPRESSIONS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 263 KEY ISLAND DRESSINGS OFFERED, BY COMPANY
- TABLE 264 ISLAND DRESSINGS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 265 NORTH AMERICA: ISLAND DRESSINGS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 266 EUROPE: ISLAND DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 267 ASIA PACIFIC: ISLAND DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 268 LATIN AMERICA: ISLAND DRESSINGS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 269 OTHER TRADITIONAL WOUND CARE PRODUCTS OFFERED, BY COMPANY
- TABLE 270 OTHER TRADITIONAL WOUND CARE PRODUCTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 271 NORTH AMERICA: OTHER TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 272 EUROPE: OTHER TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 273 ASIA PACIFIC: OTHER TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 274 LATIN AMERICA: OTHER TRADITIONAL WOUND CARE PRODUCTS MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 275 WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 276 WOUND CARE MARKET FOR CHRONIC WOUNDS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 277 WOUND CARE MARKET FOR CHRONIC WOUNDS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 278 NORTH AMERICA: WOUND CARE MARKET FOR CHRONIC WOUNDS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 279 EUROPE: WOUND CARE MARKET FOR CHRONIC WOUNDS, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 280 ASIA PACIFIC: WOUND CARE MARKET FOR CHRONIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 281 LATIN AMERICA: WOUND CARE MARKET FOR CHRONIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 282 DIABETES-RELATED HEALTHCARE EXPENDITURE, BY TERRITORY, 2024 (USD MILLION)
- TABLE 283 NUMBER OF ADULT PATIENTS WITH DIABETIC FOOT ULCERS, BY COUNTRY, 2023-2030 (THOUSANDS)
- TABLE 284 WOUND CARE MARKET FOR DIABETIC FOOT ULCERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 285 NORTH AMERICA: WOUND CARE MARKET FOR DIABETIC FOOT ULCERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 286 EUROPE: WOUND CARE MARKET FOR DIABETIC FOOT ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 287 ASIA PACIFIC: WOUND CARE MARKET FOR DIABETIC FOOT ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 288 LATIN AMERICA: WOUND CARE MARKET FOR DIABETIC FOOT ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 289 WOUND CARE MARKET FOR PRESSURE ULCERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 290 NORTH AMERICA: WOUND CARE MARKET FOR PRESSURE ULCERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 291 EUROPE: WOUND CARE MARKET FOR PRESSURE ULCERS, BY COUNTRY/ REGION, 2023-2030 (USD MILLION)
- TABLE 292 ASIA PACIFIC: WOUND CARE MARKET FOR PRESSURE ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 293 LATIN AMERICA: WOUND CARE MARKET FOR PRESSURE ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 294 WOUND CARE MARKET FOR VENOUS LEG ULCERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 295 NORTH AMERICA: WOUND CARE MARKET FOR VENOUS LEG ULCERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 296 EUROPE: WOUND CARE MARKET FOR VENOUS LEG ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 297 ASIA PACIFIC: WOUND CARE MARKET FOR VENOUS LEG ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 298 LATIN AMERICA: WOUND CARE MARKET FOR VENOUS LEG ULCERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 299 WOUND CARE MARKET FOR OTHER CHRONIC WOUNDS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 300 NORTH AMERICA: WOUND CARE MARKET FOR OTHER CHRONIC WOUNDS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 301 EUROPE: WOUND CARE MARKET FOR OTHER CHRONIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 302 ASIA PACIFIC: WOUND CARE MARKET FOR OTHER CHRONIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 303 LATIN AMERICA: WOUND CARE MARKET FOR OTHER CHRONIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 304 WOUND CARE MARKET FOR ACUTE WOUNDS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 305 WOUND CARE MARKET FOR ACUTE WOUNDS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 306 NORTH AMERICA: WOUND CARE MARKET FOR ACUTE WOUNDS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 307 EUROPE: WOUND CARE MARKET FOR ACUTE WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 308 ASIA PACIFIC: WOUND CARE MARKET FOR ACUTE WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 309 LATIN AMERICA: WOUND CARE MARKET FOR ACUTE WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 310 WOUND CARE MARKET FOR SURGICAL & TRAUMATIC WOUNDS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 311 NORTH AMERICA: WOUND CARE MARKET FOR SURGICAL & TRAUMATIC WOUNDS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 312 EUROPE: WOUND CARE MARKET FOR SURGICAL & TRAUMATIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 313 ASIA PACIFIC: WOUND CARE MARKET FOR SURGICAL & TRAUMATIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 314 LATIN AMERICA: WOUND CARE MARKET FOR SURGICAL & TRAUMATIC WOUNDS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 315 WOUND CARE MARKET FOR BURNS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 316 NORTH AMERICA: WOUND CARE MARKET FOR BURNS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 317 EUROPE: WOUND CARE MARKET FOR BURNS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 318 ASIA PACIFIC: WOUND CARE MARKET FOR BURNS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 319 LATIN AMERICA: WOUND CARE MARKET FOR BURNS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 320 WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 321 HOSPITALS PER CAPITA, BY COUNTRY, 2024
- TABLE 322 WOUND CARE MARKET FOR HOSPITALS & CLINICS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 323 WOUND CARE MARKET FOR HOSPITALS & CLINICS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 324 NORTH AMERICA: WOUND CARE MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 325 EUROPE: WOUND CARE MARKET FOR HOSPITALS & CLINICS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 326 ASIA PACIFIC: WOUND CARE MARKET FOR HOSPITALS & CLINICS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 327 LATIN AMERICA: WOUND CARE MARKET FOR HOSPITALS & CLINICS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 328 WOUND CARE MARKET FOR INPATIENT SETTINGS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 329 NORTH AMERICA: WOUND CARE MARKET FOR INPATIENT SETTINGS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 330 EUROPE: WOUND CARE MARKET FOR INPATIENT SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 331 ASIA PACIFIC: WOUND CARE MARKET FOR INPATIENT SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 332 LATIN AMERICA: WOUND CARE MARKET FOR INPATIENT SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 333 WOUND CARE MARKET FOR OUTPATIENT SETTINGS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 334 NORTH AMERICA: WOUND CARE MARKET FOR OUTPATIENT SETTINGS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 335 EUROPE: WOUND CARE MARKET FOR OUTPATIENT SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 336 ASIA PACIFIC: WOUND CARE MARKET FOR OUTPATIENT SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 337 LATIN AMERICA: WOUND CARE MARKET FOR OUTPATIENT SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 338 WOUND CARE MARKET FOR HOME CARE SETTINGS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 339 NORTH AMERICA: WOUND CARE MARKET FOR HOME CARE SETTINGS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 340 EUROPE: WOUND CARE MARKET FOR HOME CARE SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 341 ASIA PACIFIC: WOUND CARE MARKET FOR HOME CARE SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 342 LATIN AMERICA: WOUND CARE MARKET FOR HOME CARE SETTINGS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 343 WOUND CARE MARKET FOR LONG-TERM CARE FACILITIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 344 NORTH AMERICA: WOUND CARE MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 345 EUROPE: WOUND CARE MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 346 ASIA PACIFIC: WOUND CARE MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 347 LATIN AMERICA: WOUND CARE MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 348 WOUND CARE MARKET FOR OTHER END USERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 349 NORTH AMERICA: WOUND CARE MARKET FOR OTHER END USERS, 2023-2030 (USD MILLION)
- TABLE 350 EUROPE: WOUND CARE MARKET FOR OTHER END USERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 351 ASIA PACIFIC: WOUND CARE MARKET FOR OTHER END USERS, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 352 LATIN AMERICA: WOUND CARE MARKET FOR OTHER END USERS, BY COUNTRY/REGION, 2022-2030 (USD MILLION)
- TABLE 353 WOUND CARE MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 354 NORTH AMERICA: MACROECONOMIC INDICATORS
- TABLE 355 NORTH AMERICA: WOUND CARE MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 356 NORTH AMERICA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 357 NORTH AMERICA: ADVANCED WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 358 NORTH AMERICA: ADVANCED WOUND DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 359 NORTH AMERICA: FOAM DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 360 NORTH AMERICA: ADVANCED WOUND DRESSINGS MARKET, BY PROPERTY, 2023-2030 (USD MILLION)
- TABLE 361 NORTH AMERICA: DEVICES & ACCESSORIES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 362 NORTH AMERICA: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 363 NORTH AMERICA: SURGICAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 364 NORTH AMERICA: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 365 NORTH AMERICA: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 366 NORTH AMERICA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 367 NORTH AMERICA: CHRONIC WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 368 NORTH AMERICA: ACUTE WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 369 NORTH AMERICA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 370 NORTH AMERICA: HOSPITALS & CLINICS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 371 US: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 372 US: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 373 US: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 374 ESTIMATED NUMBER OF DIABETICS IN CANADA, 2024 VS. 2034
- TABLE 375 CANADA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 376 CANADA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 377 CANADA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 378 EUROPE: MACROECONOMIC INDICATORS
- TABLE 379 EUROPE: WOUND CARE MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 380 EUROPE: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 381 EUROPE: ADVANCED WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 382 EUROPE: ADVANCED WOUND DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 383 EUROPE: FOAM DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 384 EUROPE: ADVANCED WOUND DRESSINGS MARKET, BY PROPERTY, 2023-2030 (USD MILLION)
- TABLE 385 EUROPE: DEVICES & ACCESSORIES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 386 EUROPE: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 387 EUROPE: SURGICAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 388 EUROPE: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 389 EUROPE: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 390 EUROPE: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 391 EUROPE: CHRONIC WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 392 EUROPE: ACUTE WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 393 EUROPE: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 394 EUROPE: HOSPITALS & CLINICS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 395 GERMANY: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 396 GERMANY: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 397 GERMANY: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 398 FRANCE: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 399 FRANCE: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 400 FRANCE: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 401 UK: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 402 UK: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 403 UK: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 404 ITALY: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 405 ITALY: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 406 ITALY: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 407 SPAIN: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 408 SPAIN: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 409 SPAIN: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 410 RUSSIA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 411 RUSSIA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 412 RUSSIA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 413 REST OF EUROPE: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 414 REST OF EUROPE: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 415 REST OF EUROPE: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 416 ASIA PACIFIC: KEY MACROECONOMIC INDICATORS
- TABLE 417 ASIA PACIFIC: WOUND CARE MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 418 ASIA PACIFIC: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 419 ASIA PACIFIC: ADVANCED WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 420 ASIA PACIFIC: ADVANCED WOUND DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 421 ASIA PACIFIC: FOAM DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 422 ASIA PACIFIC: ADVANCED WOUND DRESSINGS MARKET, BY PROPERTY, 2023-2030 (USD MILLION)
- TABLE 423 ASIA PACIFIC: DEVICES & ACCESSORIES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 424 ASIA PACIFIC: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 425 ASIA PACIFIC: SURGICAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 426 ASIA PACIFIC: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 427 ASIA PACIFIC: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 428 ASIA PACIFIC: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 429 ASIA PACIFIC: CHRONIC WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 430 ASIA PACIFIC: ACUTE WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 431 ASIA PACIFIC: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 432 ASIA PACIFIC: HOSPITALS & CLINICS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 433 CHINA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 434 CHINA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 435 CHINA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 436 JAPAN: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 437 JAPAN: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 438 JAPAN: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 439 INDIA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 440 INDIA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 441 INDIA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 442 AUSTRALIA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 443 AUSTRALIA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 444 AUSTRALIA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 445 SOUTH KOREA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 446 SOUTH KOREA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 447 SOUTH KOREA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 448 SINGAPORE: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 449 SINGAPORE: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 450 SINGAPORE: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 451 REST OF ASIA PACIFIC: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 452 REST OF ASIA PACIFIC: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 453 REST OF ASIA PACIFIC: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 454 LATIN AMERICA: KEY MACROECONOMIC INDICATORS
- TABLE 455 LATIN AMERICA: WOUND CARE MARKET, BY COUNTRY/REGION, 2023-2030 (USD MILLION)
- TABLE 456 LATIN AMERICA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 457 LATIN AMERICA: ADVANCED WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 458 LATIN AMERICA: ADVANCED WOUND DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 459 LATIN AMERICA: FOAM DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 460 LATIN AMERICA: ADVANCED WOUND DRESSINGS MARKET, BY PROPERTY, 2023-2030 (USD MILLION)
- TABLE 461 LATIN AMERICA: DEVICES & ACCESSORIES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 462 LATIN AMERICA: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 463 LATIN AMERICA: SURGICAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 464 LATIN AMERICA: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 465 LATIN AMERICA: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 466 LATIN AMERICA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 467 LATIN AMERICA: CHRONIC WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 468 LATIN AMERICA: ACUTE WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 469 LATIN AMERICA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 470 LATIN AMERICA: HOSPITALS & CLINICS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 471 BRAZIL: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 472 BRAZIL: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 473 BRAZIL: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 474 MEXICO: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 475 MEXICO: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 476 MEXICO: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 477 REST OF LATIN AMERICA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 478 REST OF LATIN AMERICA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 479 REST OF LATIN AMERICA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 480 MIDDLE EAST & AFRICA: KEY MACROECONOMIC INDICATORS
- TABLE 481 MIDDLE EAST & AFRICA: WOUND CARE MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 482 MIDDLE EAST & AFRICA: ADVANCED WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 483 MIDDLE EAST & AFRICA: ADVANCED WOUND DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 484 MIDDLE EAST & AFRICA: FOAM DRESSINGS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 485 MIDDLE EAST & AFRICA: ADVANCED WOUND DRESSINGS MARKET, BY PROPERTY, 2023-2030 (USD MILLION)
- TABLE 486 MIDDLE EAST & AFRICA: DEVICES & ACCESSORIES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 487 MIDDLE EAST & AFRICA: BIOLOGICAL SKIN SUBSTITUTES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 488 MIDDLE EAST & AFRICA: SURGICAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 489 MIDDLE EAST & AFRICA: TISSUE ADHESIVES, SEALANTS, AND GLUES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 490 MIDDLE EAST & AFRICA: TRADITIONAL WOUND CARE PRODUCTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 491 MIDDLE EAST & AFRICA: WOUND CARE MARKET, BY WOUND TYPE, 2023-2030 (USD MILLION)
- TABLE 492 MIDDLE EAST & AFRICA: CHRONIC WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 493 MIDDLE EAST & AFRICA: ACUTE WOUND MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 494 MIDDLE EAST & AFRICA: WOUND CARE MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 495 MIDDLE EAST & AFRICA: HOSPITALS & CLINICS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 496 OVERVIEW OF STRATEGIES DEPLOYED BY KEY PLAYERS, JANUARY 2022-MAY 2025
- TABLE 497 WOUND CARE MARKET: DEGREE OF COMPETITION
- TABLE 498 WOUND CARE MARKET: REGION FOOTPRINT
- TABLE 499 WOUND CARE MARKET: PRODUCT FOOTPRINT
- TABLE 500 WOUND CARE MARKET: WOUND TYPE FOOTPRINT
- TABLE 501 WOUND CARE MARKET: END USER FOOTPRINT
- TABLE 502 WOUND CARE MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 503 WOUND CARE MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
- TABLE 504 WOUND CARE MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 505 WOUND CARE MARKET: DEALS, JANUARY 2022-MAY 2025
- TABLE 506 WOUND CARE MARKET: EXPANSIONS, JANUARY 2022-MAY 2025
- TABLE 507 WOUND CARE MARKET: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 508 SOLVENTUM: COMPANY OVERVIEW
- TABLE 509 SOLVENTUM: PRODUCTS OFFERED
- TABLE 510 SOLVENTUM: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 511 SOLVENTUM: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 512 JOHNSON & JOHNSON SERVICES, INC.: COMPANY OVERVIEW
- TABLE 513 JOHNSON & JOHNSON SERVICES, INC.: PRODUCTS OFFERED
- TABLE 514 JOHNSON & JOHNSON SERVICES, INC.: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 515 SMITH+NEPHEW: COMPANY OVERVIEW
- TABLE 516 SMITH+NEPHEW: PRODUCTS OFFERED
- TABLE 517 SMITH+NEPHEW: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 518 SMITH+NEPHEW: DEALS, JANUARY 2022-MAY 2025
- TABLE 519 SMITH+NEPHEW: EXPANSIONS, JANUARY 2022-MAY 2025
- TABLE 520 SMITH+NEPHEW: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 521 CARDINAL HEALTH: COMPANY OVERVIEW
- TABLE 522 CARDINAL HEALTH: PRODUCTS OFFERED
- TABLE 523 CARDINAL HEALTH: DEALS, JANUARY 2022-MAY 2025
- TABLE 524 CARDINAL HEALTH: EXPANSIONS, JANUARY 2022-MAY 2025
- TABLE 525 MOLNLYCKE AB: COMPANY OVERVIEW
- TABLE 526 MOLNLYCKE AB: PRODUCTS OFFERED
- TABLE 527 MOLNLYCKE AB: DEALS, JANUARY 2022-MAY 2025
- TABLE 528 MOLNLYCKE AB: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 529 CONVATEC GROUP PLC: COMPANY OVERVIEW
- TABLE 530 CONVATEC GROUP PLC: PRODUCTS OFFERED
- TABLE 531 CONVATEC GROUP PLC: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 532 CONVATEC GROUP PLC: DEALS, JANUARY 2022-MAY 2025
- TABLE 533 COLOPLAST GROUP: COMPANY OVERVIEW
- TABLE 534 COLOPLAST GROUP: PRODUCTS OFFERED
- TABLE 535 COLOPLAST GROUP: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 536 COLOPLAST GROUP: DEALS, JANUARY 2022-MAY 2025
- TABLE 537 ORGANOGENESIS INC.: COMPANY OVERVIEW
- TABLE 538 ORGANOGENESIS INC.: PRODUCTS OFFERED
- TABLE 539 ORGANOGENESIS INC.: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 540 ORGANOGENESIS INC.: EXPANSIONS, JANUARY 2022-MAY 2025
- TABLE 541 PAUL HARTMANN AG: COMPANY OVERVIEW
- TABLE 542 PAUL HARTMANN AG: PRODUCTS OFFERED
- TABLE 543 PAUL HARTMANN AG: DEALS, JANUARY 2022-MAY 2025
- TABLE 544 MIMEDX GROUP, INC.: COMPANY OVERVIEW
- TABLE 545 MIMEDX GROUP, INC.: PRODUCTS OFFERED
- TABLE 546 MIMEDX GROUP, INC.: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 547 OWENS & MINOR: COMPANY OVERVIEW
- TABLE 548 OWENS & MINOR: PRODUCTS OFFERED
- TABLE 549 OWENS & MINOR: DEALS, JANUARY 2022-JANUARY 2025
- TABLE 550 OWENS & MINOR: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 551 B. BRAUN SE: COMPANY OVERVIEW
- TABLE 552 B. BRAUN SE: PRODUCTS OFFERED
- TABLE 553 B. BRAUN SE: EXPANSIONS, JANUARY 2022-MAY 2025
- TABLE 554 B. BRAUN SE: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 555 ESSITY AKTIEBOLAG: COMPANY OVERVIEW
- TABLE 556 ESSITY AKTIEBOLAG: PRODUCTS OFFERED
- TABLE 557 ESSITY AKTLEBOLAG: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 558 INTEGRA LIFESCIENCES CORPORATION: COMPANY OVERVIEW
- TABLE 559 INTEGRA LIFESCIENCES CORPORATION: PRODUCTS OFFERED
- TABLE 560 INTEGRA LIFESCIENCES CORPORATION: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 561 INTEGRA LIFESCIENCES CORPORATION: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 562 AVERY DENNISON CORPORATION: COMPANY OVERVIEW
- TABLE 563 AVERY DENNISON CORPORATION: PRODUCTS OFFERED
- TABLE 564 AVERY DENNISON CORPORATION: EXPANSIONS, JANUARY 2022-MAY 2025
- TABLE 565 MATIV HOLDINGS, INC.: COMPANY OVERVIEW
- TABLE 566 MATIV HOLDINGS, INC.: PRODUCTS OFFERED
- TABLE 567 MATIV HOLDINGS, INC.: DEALS, JANUARY 2022-MAY 2025
- TABLE 568 BIOVENTUS: COMPANY OVERVIEW
- TABLE 569 BIOVENTUS: PRODUCTS OFFERED
- TABLE 570 BIOVENTUS: DEALS, JANUARY 2022-JANUARY 2025
- TABLE 571 BIOVENTUS: OTHER DEVELOPMENTS, JANUARY 2022-MAY 2025
- TABLE 572 ZIMMER BIOMET: COMPANY OVERVIEW
- TABLE 573 ZIMMER BIOMET: PRODUCTS OFFERED
- TABLE 574 ZIMMER BIOMET: DEALS, JANUARY 2022-MAY 2025
- TABLE 575 MEDTRONIC: COMPANY OVERVIEW
- TABLE 576 MEDTRONIC: PRODUCTS OFFERED
- TABLE 577 BAXTER: COMPANY OVERVIEW
- TABLE 578 BAXTER: PRODUCTS OFFERED
- TABLE 579 BAXTER: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-MAY 2025
- TABLE 580 LOHMANN & RAUSCHER GMBH & CO. KG: COMPANY OVERVIEW
- TABLE 581 MEDLINE INDUSTRIES, LP: COMPANY OVERVIEW
- TABLE 582 DEROYAL INDUSTRIES, INC.: COMPANY OVERVIEW
- TABLE 583 WINNER MEDICAL CO., LTD.: COMPANY OVERVIEW
- TABLE 584 ADVANCIS (UK): COMPANY OVERVIEW
- TABLE 585 MIL LABORATORIES PVT. LTD.: COMPANY OVERVIEW
- TABLE 586 PENSAR MEDICAL: COMPANY OVERVIEW
- TABLE 587 HAROMED BV: COMPANY OVERVIEW
- TABLE 588 URGO GROUP: COMPANY OVERVIEW
- TABLE 589 DIRECT HEALTHCARE GROUP: COMPANY OVERVIEW
List of Figures
- FIGURE 1 MARKET SEGMENTATION AND REGIONAL SCOPE
- FIGURE 2 WOUND CARE MARKET: RESEARCH DESIGN
- FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
- FIGURE 4 BOTTOM-UP APPROACH: COMPANY REVENUE ESTIMATION APPROACH
- FIGURE 5 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
- FIGURE 6 WOUND CARE MARKET: TOP-DOWN APPROACH
- FIGURE 7 DATA TRIANGULATION METHODOLOGY
- FIGURE 8 WOUND CARE MARKET, BY PRODUCT, 2025 VS. 2030 (USD MILLION)
- FIGURE 9 WOUND CARE MARKET, BY WOUND TYPE, 2025 VS. 2030 (USD MILLION)
- FIGURE 10 WOUND CARE MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- FIGURE 11 GEOGRAPHICAL SNAPSHOT OF WOUND CARE MARKET
- FIGURE 12 INCREASING PREVALENCE OF DIABETES TO DRIVE MARKET GROWTH
- FIGURE 13 HOSPITALS & CLINICS SEGMENT AND CHINA ACCOUNTED FOR SIGNIFICANT SHARE IN 2024
- FIGURE 14 JAPAN TO RECORD HIGHEST GROWTH RATE FROM 2025 TO 2030
- FIGURE 15 ASIA PACIFIC TO WITNESS HIGHEST GROWTH DURING FORECAST PERIOD
- FIGURE 16 WOUND CARE MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 17 TRENDS AND DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- FIGURE 18 VALUE CHAIN ANALYSIS
- FIGURE 19 SUPPLY CHAIN ANALYSIS
- FIGURE 20 ECOSYSTEM ANALYSIS
- FIGURE 21 INVESTMENT AND FUNDING SCENARIO, 2020-2025 (USD MILLION)
- FIGURE 22 WOUND CARE MARKET: PATENT ANALYSIS, JANUARY 2015-DECEMBER 2024
- FIGURE 23 PORTER'S FIVE FORCES ANALYSIS
- FIGURE 24 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY KEY PRODUCT
- FIGURE 25 KEY BUYING CRITERIA FOR KEY PRODUCTS
- FIGURE 26 IMPACT OF AI ON WOUND CARE MARKET: USE CASES
- FIGURE 27 NORTH AMERICA: WOUND CARE MARKET SNAPSHOT
- FIGURE 28 ASIA PACIFIC: WOUND CARE MARKET SNAPSHOT
- FIGURE 29 REVENUE ANALYSIS OF KEY PLAYERS, 2022-2024 (USD MILLION)
- FIGURE 30 MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
- FIGURE 31 ADVANCED WOUND CARE PRODUCTS: MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
- FIGURE 32 NEGATIVE PRESSURE WOUND THERAPY (NPWT): MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
- FIGURE 33 ADVANCED WOUND DRESSINGS: MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
- FIGURE 34 TRADITIONAL WOUND CARE PRODUCTS: MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
- FIGURE 35 RANKING OF KEY PLAYERS, 2024
- FIGURE 36 WOUND CARE MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 37 WOUND CARE MARKET: COMPANY FOOTPRINT
- FIGURE 38 WOUND CARE MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 39 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN, AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 40 EV/EBITDA OF KEY VENDORS
- FIGURE 41 BRAND/PRODUCT COMPARATIVE ANALYSIS
- FIGURE 42 SOLVENTUM: COMPANY SNAPSHOT (2024)
- FIGURE 43 JOHNSON & JOHNSON SERVICES, INC.: COMPANY SNAPSHOT (2024)
- FIGURE 44 SMITH+NEPHEW: COMPANY SNAPSHOT (2024)
- FIGURE 45 CARDINAL HEALTH: COMPANY SNAPSHOT (2024)
- FIGURE 46 MOLNLYCKE AB: COMPANY SNAPSHOT (2024)
- FIGURE 47 CONVATEC GROUP PLC: COMPANY SNAPSHOT (2024)
- FIGURE 48 COLOPLAST GROUP: COMPANY SNAPSHOT (2024)
- FIGURE 49 ORGANOGENESIS INC.: COMPANY SNAPSHOT (2024)
- FIGURE 50 PAUL HARTMANN AG: COMPANY SNAPSHOT (2024)
- FIGURE 51 MIMEDX GROUP, INC.: COMPANY SNAPSHOT (2024)
- FIGURE 52 OWENS & MINOR: COMPANY SNAPSHOT (2024)
- FIGURE 53 B. BRAUN SE: COMPANY SNAPSHOT (2024)
- FIGURE 54 ESSITY AKTIEBOLAG: COMPANY SNAPSHOT (2024)
- FIGURE 55 INTEGRA LIFESCIENCES CORPORATION: COMPANY SNAPSHOT (2024)
- FIGURE 56 AVERY DENNISON CORPORATION: COMPANY SNAPSHOT (2024)
- FIGURE 57 MATIV HOLDINGS, INC.: COMPANY SNAPSHOT (2024)
- FIGURE 58 BIOVENTUS: COMPANY SNAPSHOT (2024)
- FIGURE 59 ZIMMER BIOMET: COMPANY SNAPSHOT (2024)
- FIGURE 60 MEDTRONIC: COMPANY SNAPSHOT (2024)
- FIGURE 61 BAXTER: COMPANY SNAPSHOT (2024)







