|
市場調查報告書
商品編碼
1472399
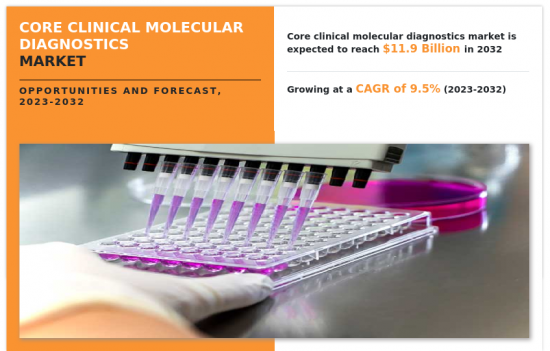
核心臨床分子診斷市場:按產品類型、技術和應用分類:2023-2032 年全球機會分析和產業預測Core Clinical Molecular Diagnostics Market By Product type, By Technique (PCR, Nucleic Acid Sequencing, Fluorescence In Situ Hybridization, Others), By Application : Global Opportunity Analysis and Industry Forecast, 2023-2032 |
||||||
2022年核心臨床分子診斷市值為48億美元,預計2032年將達119億美元,2023年至2032年複合年成長率為9.50%。
核心臨床分子診斷是指在醫學實驗室中執行的基本技術和程序。它專注於在分子層面上分析遺傳物質(DNA、RNA)和蛋白質,以幫助診斷疾病和確定治療策略。這些診斷技術包括多種技術,例如聚合酵素鏈鎖反應(PCR)、DNA定序、次世代定序(NGS)、螢光原位雜合反應(FISH) 和免疫組織化學 (IHC)。核心臨床分子診斷透過識別基因突變、感染因子和疾病相關生物標記來實現客製化治療並改善臨床結果,在個人化醫療中發揮關鍵作用。
疾病的流行和人口老化是臨床分子診斷需求不斷成長的主要驅動力。隨著人口老化,癌症、心血管疾病和神經退化性疾病等慢性和與老齡化相關的疾病的盛行率顯著增加。此外,隨著年齡的成長,免疫力會下降,使我們更容易受到感染疾病。根據美國人口普查局取得的資料,2021年,美國老年人口將占到5,400萬人,佔總人口的16.5%。此外,預計到 2050 年這一數字將增至 8,570 萬。根據美國癌症研究所的數據,乳癌的中位診斷年齡為61 歲,大腸癌的中位診斷年齡為68 歲,肺癌的中位診斷年齡為70 歲,而攝護腺癌的中位診斷年齡為66 歲。
因此,臨床分子診斷透過識別與這些疾病相關的基因突變、感染因子和生物標記物,在準確診斷特定疾病方面發揮重要作用。提供準確、及時的診斷資訊的能力使醫療保健提供者能夠實施個人化的治療策略,改善患者的治療結果,並有助於在老齡化人口中進行更有效的疾病管理。因此,為了滿足世界老齡化社會的醫療保健需求,對核心臨床分子診斷的需求預計將繼續增加。
此外,醫療保健支出的增加也是推動核心臨床分子診斷市場成長的關鍵因素。隨著全球醫療保健支出的增加,人們越來越關注提高診斷能力,以改善患者護理和治療結果。臨床核心分子診斷能力提供精確、個人化的診斷訊息,並與精準醫療日益成長的趨勢保持一致。隨著更多資源分配給醫療保健,投資 PCR、DNA定序和 NGS 等先進診斷技術的能力和意願不斷增強,正在推動市場成長。此外,隨著醫療保健系統努力最佳化資源利用並提高成本效益,核心臨床分子診斷提供的效率和精確度變得越來越有吸引力,進一步推動市場擴張。
核心臨床分子診斷市場按產品類型、技術、應用和地區細分。依產品類型,市場分為設備、試劑、軟體和服務。依技術分類,市場分為 PCR、核酸序列測定、螢光原位雜合反應(FISH) 等。依應用,市場分為感染疾病、遺傳疾病、癌症篩檢等。按地區分類,我們有北美(美國、加拿大、墨西哥)、歐洲(德國、法國、英國、義大利、西班牙其他歐洲國家地區)、亞太地區(中國、日本、印度、澳洲、韓國、其他亞太地區),在洛杉磯(巴西、哥倫比亞、阿根廷和其他洛杉磯地區)和中東和非洲(海灣合作理事會、北非和其他中東和非洲地區)進行了分析。
相關人員的主要利益
- 該報告提供了 2022 年至 2032 年核心臨床分子診斷市場分析的細分市場、當前趨勢、估計和趨勢以及動態的定量分析,並確定了核心臨床分子診斷市場的顯著市場機會。
- 我們提供市場研究以及與市場促進因素、市場限制和市場機會相關的資訊。
- 波特的五力分析揭示了買家和供應商的潛力,幫助相關人員做出利潤驅動的業務決策並加強供應商和買家網路。
- 對核心臨床分子診斷市場細分的詳細分析有助於識別市場機會。
- 每個地區的主要國家都根據其對全球市場的收益貢獻繪製了地圖。
- 市場參與者定位有助於基準化分析,並可以清楚地了解市場參與者的當前地位。
- 該報告包括對區域和全球核心臨床分子診斷市場趨勢、主要企業、細分市場、應用領域和市場成長策略的分析。
該報告可以客製化。
- 監管指引
- 根據客戶興趣加入公司簡介
- 公司簡介的擴充列表
- 歷史市場資料
目錄
第1章簡介
第 2 章執行摘要
第3章市場概況
- 市場定義和範圍
- 主要發現
- 影響因素
- 主要投資機會
- 波特五力分析
- 市場動態
- 促進因素
- 抑制因素
- 機會
第4章臨床分子診斷核心市場:依產品類型
- 概述
- 檢驗設備
- 試劑
- 軟體和服務
第5章核心臨床分子診斷市場:依技術分類
- 概述
- PCR法
- 核酸序列測定
- 螢光原位雜合反應(FISH)
- 其他
第6章核心臨床分子診斷市場:依應用分類
- 概述
- 感染疾病
- 遺傳疾病
- 癌症篩檢
- 其他
第7章核心臨床分子診斷市場:按地區
- 概述
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 法國
- 英國
- 義大利
- 西班牙
- 其他
- 亞太地區
- 日本
- 中國
- 印度
- 澳洲
- 韓國
- 其他
- 拉丁美洲
- 巴西
- 哥倫比亞
- 阿根廷
- 其他拉丁美洲
- 中東/非洲
- Gcc
- 南非
- 北非
- 其他
第8章 競爭格局
- 介紹
- 關鍵成功策略
- 10家主要企業產品圖譜
- 競爭對手儀表板
- 競爭熱圖
- 2022年主要企業定位
第9章 公司簡介
- F. Hoffmann La Roche
- Novartis AG
- Abbott Laboratories
- Becton Dickinson & Company
- Siemens Healthineers AG
- Agilent Technologies, Inc.
- Hologic, Inc.
- BioMerieux SA
- Qiagen NV.
- cepheid ab
The core clinical molecular diagnostics market was valued at $4.8 billion in 2022 and is estimated to reach $11.9 billion by 2032, exhibiting a CAGR of 9.50% from 2023 to 2032. Core clinical molecular diagnostics refer to essential techniques and procedures conducted in medical laboratories. They focus on analyzing genetic material (DNA, RNA) and proteins at the molecular level to diagnose diseases and guide treatment decisions. These diagnostics encompass various methodologies such as Polymerase Chain Reaction (PCR), DNA sequencing, Next-Generation Sequencing (NGS), Fluorescence In Situ Hybridization (FISH), and immunohistochemistry (IHC). Core clinical molecular diagnostics play a crucial role in personalized medicine by identifying genetic variations, infectious agents, and biomarkers associated with diseases, enabling tailored patient care and improving clinical outcomes.

The increasing prevalence of diseases and the aging population are major driving forces behind the growing demand for core clinical molecular diagnostics. As populations age, the incidence of chronic and age-related diseases such as cancer, cardiovascular diseases, and neurodegenerative disorders rises significantly. Additionally, the aging demographic is more susceptible to infectious diseases due to weakened immune systems. According to data obtained from the U.S. Census Bureau, in 2021, elderly population in the U.S. accounted for 16.5% of the total population, which is 54 million. Moreover, this number is anticipated to increase to 85.7 million by 2050. In addition, according to the National Cancer Institute, the median age for diagnosis is 61 years for breast cancer, 68 years for colorectal cancer, 70 years for lung cancer, and 66 years for prostate cancer.
Thus, clinical molecular diagnostics play a critical role in accurately diagnosing these conditions by identifying genetic variations, infectious agents, and biomarkers associated with specific diseases. Their ability to provide precise and timely diagnostic information enables healthcare providers to implement personalized treatment strategies, improving patient outcomes and contributing to more effective disease management in an aging population. As such, the demand for core clinical molecular diagnostics is expected to continue increasing to meet the healthcare needs of aging populations worldwide.
In addition, the rising expenditure in healthcare is indeed a significant driver propelling the growth of the core clinical molecular diagnostics market. As healthcare spending increases globally, there's a greater emphasis on improving diagnostic capabilities to enhance patient care and outcomes. The ability of clinical core molecular diagnostics offers precise and personalized diagnostic information, which aligns well with the growing trend towards precision medicine. With more resources being allocated to healthcare, there's an increased ability and willingness to invest in advanced diagnostic technologies such as PCR, DNA sequencing, and NGS, driving market growth. Additionally, as healthcare systems strive to optimize resource utilization and improve cost-effectiveness, the efficiency and accuracy offered by core clinical molecular diagnostics become increasingly attractive, further fueling market expansion.
The core clinical molecular diagnostics market is segmented on the basis of product type, technique, application, and region. On the basis of product type, the market is segmented into Instruments, reagents and software & services. On the basis of technique, the market is segmented into PCR, nucleic acid sequencing, fluorescence in situ hybridization (FISH), and others. On the basis of application, the market is segmented into infectious diseases, genetic disorders, cancer screening, and others. Region-wise, the market is analyzed across North America (U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, India, Australia, South Korea, and rest of Asia-Pacific), and LA (Brazil, Colombia, Argentina, and rest of LA) and MEA (GCC, South Africa, North Africa and rest of MEA).
The major key players that operate in the core clinical molecular diagnostics market are F. Hoffmann-La Roche Ltd., Novartis, Abbott Laboratories, Becton, Dickinson and Company, Siemens Healthineers, Agilent Technologies, Hologic, Inc. Bio-Rad Laboratories, Inc., Qiagen and bioMerieux. The key players have adopted product launch, product development and product approval as the key strategy to expand their product portfolio.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the core clinical molecular diagnostics market analysis from 2022 to 2032 to identify the prevailing core clinical molecular diagnostics market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the core clinical molecular diagnostics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global core clinical molecular diagnostics market trends, key players, market segments, application areas, and market growth strategies.
Additional benefits you will get with this purchase are:
- Quarterly Update and* (only available with a corporate license, on listed price)
- 5 additional Company Profile of client Choice pre- or Post-purchase, as a free update.
- Free Upcoming Version on the Purchase of Five and Enterprise User License.
- 16 analyst hours of support* (post-purchase, if you find additional data requirements upon review of the report, you may receive support amounting to 16 analyst hours to solve questions, and post-sale queries)
- 15% Free Customization* (in case the scope or segment of the report does not match your requirements, 15% is equivalent to 3 working days of free work, applicable once)
- Free data Pack on the Five and Enterprise User License. (Excel version of the report)
- Free Updated report if the report is 6-12 months old or older.
- 24-hour priority response*
- Free Industry updates and white papers.
Possible Customization with this report (with additional cost and timeline, please talk to the sales executive to know more)
- Regulatory Guidelines
- Additional company profiles with specific to client's interest
- Expanded list for Company Profiles
- Historic market data
Key Market Segments
By Product type
- Instruments
- Reagents
- Software and Services
By Technique
- PCR
- Nucleic Acid Sequencing
- Fluorescence In Situ Hybridization (FISH)
- Others
By Application
- Infectious Disease
- Genetic disorder
- Cancer Screening
- Others
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
- Latin America
- Brazil
- Colombia
- Argentina
- Rest Of La
- Middle East and Africa
- Gcc
- South Africa
- North Africa
- Rest Of Mea
Key Market Players:
- F. Hoffmann La Roche
- Novartis AG
- Abbott Laboratories
- Becton Dickinson & Company
- Siemens Healthineers AG
- Agilent Technologies, Inc.
- Hologic, Inc.
- BioMerieux SA
- Qiagen NV.
- cepheid ab
TABLE OF CONTENTS
CHAPTER 1: INTRODUCTION
- 1.1. Report description
- 1.2. Key market segments
- 1.3. Key benefits to the stakeholders
- 1.4. Research methodology
- 1.4.1. Primary research
- 1.4.2. Secondary research
- 1.4.3. Analyst tools and models
CHAPTER 2: EXECUTIVE SUMMARY
- 2.1. CXO perspective
CHAPTER 3: MARKET OVERVIEW
- 3.1. Market definition and scope
- 3.2. Key findings
- 3.2.1. Top impacting factors
- 3.2.2. Top investment pockets
- 3.3. Porter's five forces analysis
- 3.4. Market dynamics
- 3.4.1. Drivers
- 3.4.2. Restraints
- 3.4.3. Opportunities
CHAPTER 4: CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE
- 4.1. Overview
- 4.1.1. Market size and forecast
- 4.2. Instruments
- 4.2.1. Key market trends, growth factors and opportunities
- 4.2.2. Market size and forecast, by region
- 4.2.3. Market share analysis by country
- 4.3. Reagents
- 4.3.1. Key market trends, growth factors and opportunities
- 4.3.2. Market size and forecast, by region
- 4.3.3. Market share analysis by country
- 4.4. Software and Services
- 4.4.1. Key market trends, growth factors and opportunities
- 4.4.2. Market size and forecast, by region
- 4.4.3. Market share analysis by country
CHAPTER 5: CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE
- 5.1. Overview
- 5.1.1. Market size and forecast
- 5.2. PCR
- 5.2.1. Key market trends, growth factors and opportunities
- 5.2.2. Market size and forecast, by region
- 5.2.3. Market share analysis by country
- 5.3. Nucleic Acid Sequencing
- 5.3.1. Key market trends, growth factors and opportunities
- 5.3.2. Market size and forecast, by region
- 5.3.3. Market share analysis by country
- 5.4. Fluorescence In Situ Hybridization (FISH)
- 5.4.1. Key market trends, growth factors and opportunities
- 5.4.2. Market size and forecast, by region
- 5.4.3. Market share analysis by country
- 5.5. Others
- 5.5.1. Key market trends, growth factors and opportunities
- 5.5.2. Market size and forecast, by region
- 5.5.3. Market share analysis by country
CHAPTER 6: CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION
- 6.1. Overview
- 6.1.1. Market size and forecast
- 6.2. Infectious Disease
- 6.2.1. Key market trends, growth factors and opportunities
- 6.2.2. Market size and forecast, by region
- 6.2.3. Market share analysis by country
- 6.3. Genetic disorder
- 6.3.1. Key market trends, growth factors and opportunities
- 6.3.2. Market size and forecast, by region
- 6.3.3. Market share analysis by country
- 6.4. Cancer Screening
- 6.4.1. Key market trends, growth factors and opportunities
- 6.4.2. Market size and forecast, by region
- 6.4.3. Market share analysis by country
- 6.5. Others
- 6.5.1. Key market trends, growth factors and opportunities
- 6.5.2. Market size and forecast, by region
- 6.5.3. Market share analysis by country
CHAPTER 7: CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY REGION
- 7.1. Overview
- 7.1.1. Market size and forecast By Region
- 7.2. North America
- 7.2.1. Key market trends, growth factors and opportunities
- 7.2.2. Market size and forecast, by Product type
- 7.2.3. Market size and forecast, by Technique
- 7.2.4. Market size and forecast, by Application
- 7.2.5. Market size and forecast, by country
- 7.2.5.1. U.S.
- 7.2.5.1.1. Market size and forecast, by Product type
- 7.2.5.1.2. Market size and forecast, by Technique
- 7.2.5.1.3. Market size and forecast, by Application
- 7.2.5.2. Canada
- 7.2.5.2.1. Market size and forecast, by Product type
- 7.2.5.2.2. Market size and forecast, by Technique
- 7.2.5.2.3. Market size and forecast, by Application
- 7.2.5.3. Mexico
- 7.2.5.3.1. Market size and forecast, by Product type
- 7.2.5.3.2. Market size and forecast, by Technique
- 7.2.5.3.3. Market size and forecast, by Application
- 7.3. Europe
- 7.3.1. Key market trends, growth factors and opportunities
- 7.3.2. Market size and forecast, by Product type
- 7.3.3. Market size and forecast, by Technique
- 7.3.4. Market size and forecast, by Application
- 7.3.5. Market size and forecast, by country
- 7.3.5.1. Germany
- 7.3.5.1.1. Market size and forecast, by Product type
- 7.3.5.1.2. Market size and forecast, by Technique
- 7.3.5.1.3. Market size and forecast, by Application
- 7.3.5.2. France
- 7.3.5.2.1. Market size and forecast, by Product type
- 7.3.5.2.2. Market size and forecast, by Technique
- 7.3.5.2.3. Market size and forecast, by Application
- 7.3.5.3. UK
- 7.3.5.3.1. Market size and forecast, by Product type
- 7.3.5.3.2. Market size and forecast, by Technique
- 7.3.5.3.3. Market size and forecast, by Application
- 7.3.5.4. Italy
- 7.3.5.4.1. Market size and forecast, by Product type
- 7.3.5.4.2. Market size and forecast, by Technique
- 7.3.5.4.3. Market size and forecast, by Application
- 7.3.5.5. Spain
- 7.3.5.5.1. Market size and forecast, by Product type
- 7.3.5.5.2. Market size and forecast, by Technique
- 7.3.5.5.3. Market size and forecast, by Application
- 7.3.5.6. Rest of Europe
- 7.3.5.6.1. Market size and forecast, by Product type
- 7.3.5.6.2. Market size and forecast, by Technique
- 7.3.5.6.3. Market size and forecast, by Application
- 7.4. Asia-Pacific
- 7.4.1. Key market trends, growth factors and opportunities
- 7.4.2. Market size and forecast, by Product type
- 7.4.3. Market size and forecast, by Technique
- 7.4.4. Market size and forecast, by Application
- 7.4.5. Market size and forecast, by country
- 7.4.5.1. Japan
- 7.4.5.1.1. Market size and forecast, by Product type
- 7.4.5.1.2. Market size and forecast, by Technique
- 7.4.5.1.3. Market size and forecast, by Application
- 7.4.5.2. China
- 7.4.5.2.1. Market size and forecast, by Product type
- 7.4.5.2.2. Market size and forecast, by Technique
- 7.4.5.2.3. Market size and forecast, by Application
- 7.4.5.3. India
- 7.4.5.3.1. Market size and forecast, by Product type
- 7.4.5.3.2. Market size and forecast, by Technique
- 7.4.5.3.3. Market size and forecast, by Application
- 7.4.5.4. Australia
- 7.4.5.4.1. Market size and forecast, by Product type
- 7.4.5.4.2. Market size and forecast, by Technique
- 7.4.5.4.3. Market size and forecast, by Application
- 7.4.5.5. South Korea
- 7.4.5.5.1. Market size and forecast, by Product type
- 7.4.5.5.2. Market size and forecast, by Technique
- 7.4.5.5.3. Market size and forecast, by Application
- 7.4.5.6. Rest of Asia-Pacific
- 7.4.5.6.1. Market size and forecast, by Product type
- 7.4.5.6.2. Market size and forecast, by Technique
- 7.4.5.6.3. Market size and forecast, by Application
- 7.5. Latin America
- 7.5.1. Key market trends, growth factors and opportunities
- 7.5.2. Market size and forecast, by Product type
- 7.5.3. Market size and forecast, by Technique
- 7.5.4. Market size and forecast, by Application
- 7.5.5. Market size and forecast, by country
- 7.5.5.1. Brazil
- 7.5.5.1.1. Market size and forecast, by Product type
- 7.5.5.1.2. Market size and forecast, by Technique
- 7.5.5.1.3. Market size and forecast, by Application
- 7.5.5.2. Colombia
- 7.5.5.2.1. Market size and forecast, by Product type
- 7.5.5.2.2. Market size and forecast, by Technique
- 7.5.5.2.3. Market size and forecast, by Application
- 7.5.5.3. Argentina
- 7.5.5.3.1. Market size and forecast, by Product type
- 7.5.5.3.2. Market size and forecast, by Technique
- 7.5.5.3.3. Market size and forecast, by Application
- 7.5.5.4. Rest Of La
- 7.5.5.4.1. Market size and forecast, by Product type
- 7.5.5.4.2. Market size and forecast, by Technique
- 7.5.5.4.3. Market size and forecast, by Application
- 7.6. Middle East and Africa
- 7.6.1. Key market trends, growth factors and opportunities
- 7.6.2. Market size and forecast, by Product type
- 7.6.3. Market size and forecast, by Technique
- 7.6.4. Market size and forecast, by Application
- 7.6.5. Market size and forecast, by country
- 7.6.5.1. Gcc
- 7.6.5.1.1. Market size and forecast, by Product type
- 7.6.5.1.2. Market size and forecast, by Technique
- 7.6.5.1.3. Market size and forecast, by Application
- 7.6.5.2. South Africa
- 7.6.5.2.1. Market size and forecast, by Product type
- 7.6.5.2.2. Market size and forecast, by Technique
- 7.6.5.2.3. Market size and forecast, by Application
- 7.6.5.3. North Africa
- 7.6.5.3.1. Market size and forecast, by Product type
- 7.6.5.3.2. Market size and forecast, by Technique
- 7.6.5.3.3. Market size and forecast, by Application
- 7.6.5.4. Rest Of Mea
- 7.6.5.4.1. Market size and forecast, by Product type
- 7.6.5.4.2. Market size and forecast, by Technique
- 7.6.5.4.3. Market size and forecast, by Application
CHAPTER 8: COMPETITIVE LANDSCAPE
- 8.1. Introduction
- 8.2. Top winning strategies
- 8.3. Product mapping of top 10 player
- 8.4. Competitive dashboard
- 8.5. Competitive heatmap
- 8.6. Top player positioning, 2022
CHAPTER 9: COMPANY PROFILES
- 9.1. F. Hoffmann La Roche
- 9.1.1. Company overview
- 9.1.2. Key executives
- 9.1.3. Company snapshot
- 9.1.4. Operating business segments
- 9.1.5. Product portfolio
- 9.1.6. Business performance
- 9.1.7. Key strategic moves and developments
- 9.2. Novartis AG
- 9.2.1. Company overview
- 9.2.2. Key executives
- 9.2.3. Company snapshot
- 9.2.4. Operating business segments
- 9.2.5. Product portfolio
- 9.2.6. Business performance
- 9.2.7. Key strategic moves and developments
- 9.3. Abbott Laboratories
- 9.3.1. Company overview
- 9.3.2. Key executives
- 9.3.3. Company snapshot
- 9.3.4. Operating business segments
- 9.3.5. Product portfolio
- 9.3.6. Business performance
- 9.3.7. Key strategic moves and developments
- 9.4. Becton Dickinson & Company
- 9.4.1. Company overview
- 9.4.2. Key executives
- 9.4.3. Company snapshot
- 9.4.4. Operating business segments
- 9.4.5. Product portfolio
- 9.4.6. Business performance
- 9.4.7. Key strategic moves and developments
- 9.5. Siemens Healthineers AG
- 9.5.1. Company overview
- 9.5.2. Key executives
- 9.5.3. Company snapshot
- 9.5.4. Operating business segments
- 9.5.5. Product portfolio
- 9.5.6. Business performance
- 9.5.7. Key strategic moves and developments
- 9.6. Agilent Technologies, Inc.
- 9.6.1. Company overview
- 9.6.2. Key executives
- 9.6.3. Company snapshot
- 9.6.4. Operating business segments
- 9.6.5. Product portfolio
- 9.6.6. Business performance
- 9.6.7. Key strategic moves and developments
- 9.7. Hologic, Inc.
- 9.7.1. Company overview
- 9.7.2. Key executives
- 9.7.3. Company snapshot
- 9.7.4. Operating business segments
- 9.7.5. Product portfolio
- 9.7.6. Business performance
- 9.7.7. Key strategic moves and developments
- 9.8. BioMerieux SA
- 9.8.1. Company overview
- 9.8.2. Key executives
- 9.8.3. Company snapshot
- 9.8.4. Operating business segments
- 9.8.5. Product portfolio
- 9.8.6. Business performance
- 9.8.7. Key strategic moves and developments
- 9.9. Qiagen NV.
- 9.9.1. Company overview
- 9.9.2. Key executives
- 9.9.3. Company snapshot
- 9.9.4. Operating business segments
- 9.9.5. Product portfolio
- 9.9.6. Business performance
- 9.9.7. Key strategic moves and developments
- 9.10. cepheid ab
- 9.10.1. Company overview
- 9.10.2. Key executives
- 9.10.3. Company snapshot
- 9.10.4. Operating business segments
- 9.10.5. Product portfolio
- 9.10.6. Business performance
- 9.10.7. Key strategic moves and developments
LIST OF TABLES
- TABLE 01. GLOBAL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 02. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR INSTRUMENTS, BY REGION, 2022-2032 ($MILLION)
- TABLE 03. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR REAGENTS, BY REGION, 2022-2032 ($MILLION)
- TABLE 04. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR SOFTWARE AND SERVICES, BY REGION, 2022-2032 ($MILLION)
- TABLE 05. GLOBAL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 06. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR PCR, BY REGION, 2022-2032 ($MILLION)
- TABLE 07. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR NUCLEIC ACID SEQUENCING, BY REGION, 2022-2032 ($MILLION)
- TABLE 08. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY REGION, 2022-2032 ($MILLION)
- TABLE 09. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR OTHERS, BY REGION, 2022-2032 ($MILLION)
- TABLE 10. GLOBAL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 11. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR INFECTIOUS DISEASE, BY REGION, 2022-2032 ($MILLION)
- TABLE 12. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR GENETIC DISORDER, BY REGION, 2022-2032 ($MILLION)
- TABLE 13. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR CANCER SCREENING, BY REGION, 2022-2032 ($MILLION)
- TABLE 14. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR OTHERS, BY REGION, 2022-2032 ($MILLION)
- TABLE 15. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY REGION, 2022-2032 ($MILLION)
- TABLE 16. NORTH AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 17. NORTH AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 18. NORTH AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 19. NORTH AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 20. U.S. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 21. U.S. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 22. U.S. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 23. CANADA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 24. CANADA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 25. CANADA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 26. MEXICO CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 27. MEXICO CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 28. MEXICO CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 29. EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 30. EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 31. EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 32. EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 33. GERMANY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 34. GERMANY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 35. GERMANY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 36. FRANCE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 37. FRANCE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 38. FRANCE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 39. UK CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 40. UK CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 41. UK CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 42. ITALY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 43. ITALY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 44. ITALY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 45. SPAIN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 46. SPAIN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 47. SPAIN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 48. REST OF EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 49. REST OF EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 50. REST OF EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 51. ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 52. ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 53. ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 54. ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 55. JAPAN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 56. JAPAN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 57. JAPAN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 58. CHINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 59. CHINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 60. CHINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 61. INDIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 62. INDIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 63. INDIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 64. AUSTRALIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 65. AUSTRALIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 66. AUSTRALIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 67. SOUTH KOREA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 68. SOUTH KOREA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 69. SOUTH KOREA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 70. REST OF ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 71. REST OF ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 72. REST OF ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 73. LATIN AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 74. LATIN AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 75. LATIN AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 76. LATIN AMERICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 77. BRAZIL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 78. BRAZIL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 79. BRAZIL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 80. COLOMBIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 81. COLOMBIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 82. COLOMBIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 83. ARGENTINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 84. ARGENTINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 85. ARGENTINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 86. REST OF LA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 87. REST OF LA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 88. REST OF LA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 89. MIDDLE EAST AND AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 90. MIDDLE EAST AND AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 91. MIDDLE EAST AND AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 92. MIDDLE EAST AND AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 93. GCC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 94. GCC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 95. GCC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 96. SOUTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 97. SOUTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 98. SOUTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 99. NORTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 100. NORTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 101. NORTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 102. REST OF MEA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 103. REST OF MEA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022-2032 ($MILLION)
- TABLE 104. REST OF MEA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 105. F. HOFFMANN LA ROCHE: KEY EXECUTIVES
- TABLE 106. F. HOFFMANN LA ROCHE: COMPANY SNAPSHOT
- TABLE 107. F. HOFFMANN LA ROCHE: PRODUCT SEGMENTS
- TABLE 108. F. HOFFMANN LA ROCHE: SERVICE SEGMENTS
- TABLE 109. F. HOFFMANN LA ROCHE: PRODUCT PORTFOLIO
- TABLE 110. F. HOFFMANN LA ROCHE: KEY STRATERGIES
- TABLE 111. NOVARTIS AG: KEY EXECUTIVES
- TABLE 112. NOVARTIS AG: COMPANY SNAPSHOT
- TABLE 113. NOVARTIS AG: PRODUCT SEGMENTS
- TABLE 114. NOVARTIS AG: SERVICE SEGMENTS
- TABLE 115. NOVARTIS AG: PRODUCT PORTFOLIO
- TABLE 116. NOVARTIS AG: KEY STRATERGIES
- TABLE 117. ABBOTT LABORATORIES: KEY EXECUTIVES
- TABLE 118. ABBOTT LABORATORIES: COMPANY SNAPSHOT
- TABLE 119. ABBOTT LABORATORIES: PRODUCT SEGMENTS
- TABLE 120. ABBOTT LABORATORIES: SERVICE SEGMENTS
- TABLE 121. ABBOTT LABORATORIES: PRODUCT PORTFOLIO
- TABLE 122. ABBOTT LABORATORIES: KEY STRATERGIES
- TABLE 123. BECTON DICKINSON & COMPANY: KEY EXECUTIVES
- TABLE 124. BECTON DICKINSON & COMPANY: COMPANY SNAPSHOT
- TABLE 125. BECTON DICKINSON & COMPANY: PRODUCT SEGMENTS
- TABLE 126. BECTON DICKINSON & COMPANY: SERVICE SEGMENTS
- TABLE 127. BECTON DICKINSON & COMPANY: PRODUCT PORTFOLIO
- TABLE 128. BECTON DICKINSON & COMPANY: KEY STRATERGIES
- TABLE 129. SIEMENS HEALTHINEERS AG: KEY EXECUTIVES
- TABLE 130. SIEMENS HEALTHINEERS AG: COMPANY SNAPSHOT
- TABLE 131. SIEMENS HEALTHINEERS AG: PRODUCT SEGMENTS
- TABLE 132. SIEMENS HEALTHINEERS AG: SERVICE SEGMENTS
- TABLE 133. SIEMENS HEALTHINEERS AG: PRODUCT PORTFOLIO
- TABLE 134. SIEMENS HEALTHINEERS AG: KEY STRATERGIES
- TABLE 135. AGILENT TECHNOLOGIES, INC.: KEY EXECUTIVES
- TABLE 136. AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT
- TABLE 137. AGILENT TECHNOLOGIES, INC.: PRODUCT SEGMENTS
- TABLE 138. AGILENT TECHNOLOGIES, INC.: SERVICE SEGMENTS
- TABLE 139. AGILENT TECHNOLOGIES, INC.: PRODUCT PORTFOLIO
- TABLE 140. AGILENT TECHNOLOGIES, INC.: KEY STRATERGIES
- TABLE 141. HOLOGIC, INC.: KEY EXECUTIVES
- TABLE 142. HOLOGIC, INC.: COMPANY SNAPSHOT
- TABLE 143. HOLOGIC, INC.: PRODUCT SEGMENTS
- TABLE 144. HOLOGIC, INC.: SERVICE SEGMENTS
- TABLE 145. HOLOGIC, INC.: PRODUCT PORTFOLIO
- TABLE 146. HOLOGIC, INC.: KEY STRATERGIES
- TABLE 147. BIOMERIEUX SA: KEY EXECUTIVES
- TABLE 148. BIOMERIEUX SA: COMPANY SNAPSHOT
- TABLE 149. BIOMERIEUX SA: PRODUCT SEGMENTS
- TABLE 150. BIOMERIEUX SA: SERVICE SEGMENTS
- TABLE 151. BIOMERIEUX SA: PRODUCT PORTFOLIO
- TABLE 152. BIOMERIEUX SA: KEY STRATERGIES
- TABLE 153. QIAGEN NV.: KEY EXECUTIVES
- TABLE 154. QIAGEN NV.: COMPANY SNAPSHOT
- TABLE 155. QIAGEN NV.: PRODUCT SEGMENTS
- TABLE 156. QIAGEN NV.: SERVICE SEGMENTS
- TABLE 157. QIAGEN NV.: PRODUCT PORTFOLIO
- TABLE 158. QIAGEN NV.: KEY STRATERGIES
- TABLE 159. CEPHEID AB: KEY EXECUTIVES
- TABLE 160. CEPHEID AB: COMPANY SNAPSHOT
- TABLE 161. CEPHEID AB: PRODUCT SEGMENTS
- TABLE 162. CEPHEID AB: SERVICE SEGMENTS
- TABLE 163. CEPHEID AB: PRODUCT PORTFOLIO
- TABLE 164. CEPHEID AB: KEY STRATERGIES
LIST OF FIGURES
- FIGURE 01. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032
- FIGURE 02. SEGMENTATION OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET,2022-2032
- FIGURE 03. TOP IMPACTING FACTORS IN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET
- FIGURE 04. TOP INVESTMENT POCKETS IN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET (2023-2032)
- FIGURE 05. BARGAINING POWER OF SUPPLIERS
- FIGURE 06. BARGAINING POWER OF BUYERS
- FIGURE 07. THREAT OF SUBSTITUTION
- FIGURE 08. THREAT OF SUBSTITUTION
- FIGURE 09. COMPETITIVE RIVALRY
- FIGURE 10. GLOBAL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET:DRIVERS, RESTRAINTS AND OPPORTUNITIES
- FIGURE 11. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2022 AND 2032(%)
- FIGURE 12. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR INSTRUMENTS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 13. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR REAGENTS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 14. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR SOFTWARE AND SERVICES, BY COUNTRY 2022 AND 2032(%)
- FIGURE 15. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2022 AND 2032(%)
- FIGURE 16. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR PCR, BY COUNTRY 2022 AND 2032(%)
- FIGURE 17. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR NUCLEIC ACID SEQUENCING, BY COUNTRY 2022 AND 2032(%)
- FIGURE 18. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY COUNTRY 2022 AND 2032(%)
- FIGURE 19. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR OTHERS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 20. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2022 AND 2032(%)
- FIGURE 21. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR INFECTIOUS DISEASE, BY COUNTRY 2022 AND 2032(%)
- FIGURE 22. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR GENETIC DISORDER, BY COUNTRY 2022 AND 2032(%)
- FIGURE 23. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR CANCER SCREENING, BY COUNTRY 2022 AND 2032(%)
- FIGURE 24. COMPARATIVE SHARE ANALYSIS OF CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET FOR OTHERS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 25. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET BY REGION, 2022 AND 2032(%)
- FIGURE 26. U.S. CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 27. CANADA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 28. MEXICO CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 29. GERMANY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 30. FRANCE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 31. UK CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 32. ITALY CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 33. SPAIN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 34. REST OF EUROPE CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 35. JAPAN CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 36. CHINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 37. INDIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 38. AUSTRALIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 39. SOUTH KOREA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 40. REST OF ASIA-PACIFIC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 41. BRAZIL CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 42. COLOMBIA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 43. ARGENTINA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 44. REST OF LA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 45. GCC CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 46. SOUTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 47. NORTH AFRICA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 48. REST OF MEA CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET, 2022-2032 ($MILLION)
- FIGURE 49. TOP WINNING STRATEGIES, BY YEAR
- FIGURE 50. TOP WINNING STRATEGIES, BY DEVELOPMENT
- FIGURE 51. TOP WINNING STRATEGIES, BY COMPANY
- FIGURE 52. PRODUCT MAPPING OF TOP 10 PLAYERS
- FIGURE 53. COMPETITIVE DASHBOARD
- FIGURE 54. COMPETITIVE HEATMAP: CORE CLINICAL MOLECULAR DIAGNOSTICS MARKET
- FIGURE 55. TOP PLAYER POSITIONING, 2022