 |
市場調查報告書
商品編碼
1891249
外骨骼市場:產業趨勢及全球預測(至 2035 年)-依身體部位、運動模式、外骨骼形態、移動性別及主要地區劃分Exoskeleton Market: Industry Trends and Global Forecasts, Till 2035 Distribution by Body Part Covered, Mode of Operation, Form of Exoskeleton, Mobility and Key Geographical Regions |
||||||
外骨骼市場:概述
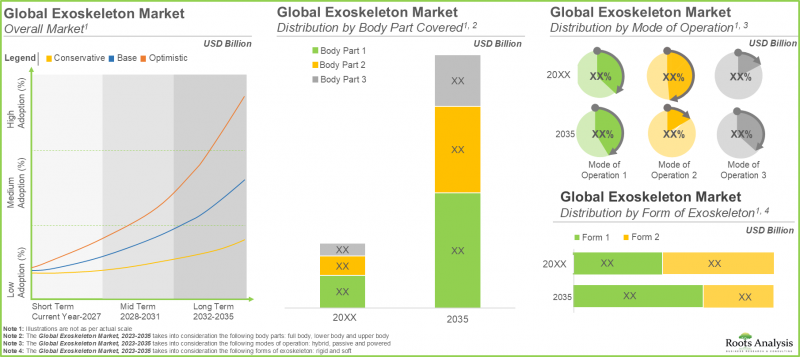
全球外骨骼市場預計將以 22.4% 的複合年增長率成長,從目前的 29 億美元成長至 2035 年的 203 億美元。
市場規模與機會分析依下列參數劃分:
目標身體部位:
- 上肢
- 下肢
- 全身
操作方式模式:
- 動力型
- 被動型
- 混合型
形式:
- 動力型
- 非動力型
- 混合型
移動性
- 固定/支撐
- 移動型
最終使用者
- 患者
- 醫療保健提供者
主要地區
- 北美
- 歐洲
- 亞太地區
- 世界其他地區
醫用外骨骼市場:成長與趨勢
近年來,機器人技術取得了顯著進步,提高了外骨骼在各個領域輔助和支持人類的實用性。尤其是在醫療領域,外骨骼展現出巨大的潛力,能夠為病人和醫護人員提供支持。然而,儘管經過數十年的研究,並擁有眾多潛在應用,但由於其他移動輔助系統和義肢設備的競爭,外骨骼尚未成為利益相關者的主流選擇,這些設備在臨床和家庭環境中也經常使用。
在醫療領域,外骨骼主要用於脊髓損傷(SCI)、中風、神經系統疾病或老年相關殘疾患者的復健、步態訓練和移動輔助。此外,亞太地區等地區的人口結構變化正在推動對改善移動性和老年護理的需求。據報道,在美國一家診所,外骨骼使復健效果提高了23%。
為了確保外骨骼的廣泛應用,開發人員正致力於設計滿足實際需求的外骨骼。為了解決成本效益和舒適度等問題,他們正在積極探索創新方法,旨在創造更自然、更貼合人體的 "第二層皮膚" 解決方案,而不是笨重的機器人外骨骼。 此外,目前正在努力將人工智慧 (AI) 和機器學習融入人機互動中,使設備能夠透過適當的控制演算法識別使用者意圖。同時,利用虛擬實境 (VR)、擴增實境 (AR)、客製化遊戲或這些技術的組合進行創新,旨在透過提高患者參與度和保持其持續完成指定練習的積極性來改進機器人輔助治療。隨著技術的進步和該領域的持續發展,外骨骼有望在未來幾年擁有巨大的潛力。
外骨骼市場:關鍵洞察
本報告深入分析了當前外骨骼市場的現狀,並指出了該行業的潛在成長機會。主要發現包括:
- 目前已有超過 200 種外骨骼產品上市或正在研發中,旨在幫助行動不便的患者,並降低護理人員在照顧這些患者時發生肌肉骨骼損傷的風險。
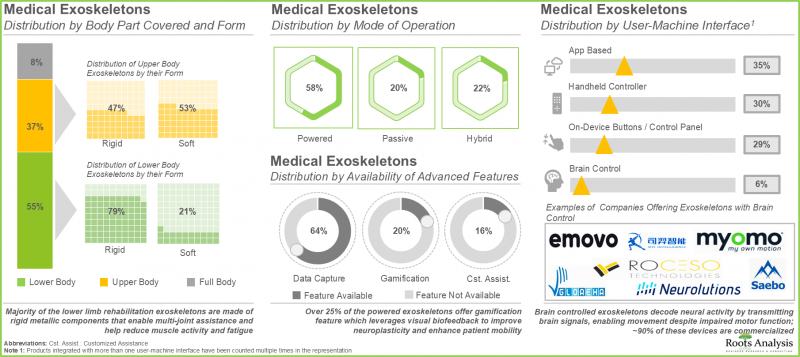
- 超過100種醫用外骨骼(包括剛性和軟性)覆蓋身體的各個部位,通常由電池供電,為感測器和執行器供電。其中超過60%的外骨骼能夠捕捉患者的運動數據。
- 醫用外骨骼的開發商遍佈全球,其中超過40%位於歐洲,主要集中在德國和瑞士等國家。
- 由於患者對醫用外骨骼的需求不斷增長,該領域已建立了多個策略合作夥伴關係。其中大部分合作夥伴關係(26%)是分銷協議。
- 醫療外骨骼開發商正致力於在其產品組合中融入先進功能,同時降低成本,旨在提高用戶採納率並最大化投資回報率 (ROI)。
- 自 2016 年以來,已有約 3800 項與外骨骼相關的專利申請和授權,這表明該領域的研究人員付出了巨大的努力。
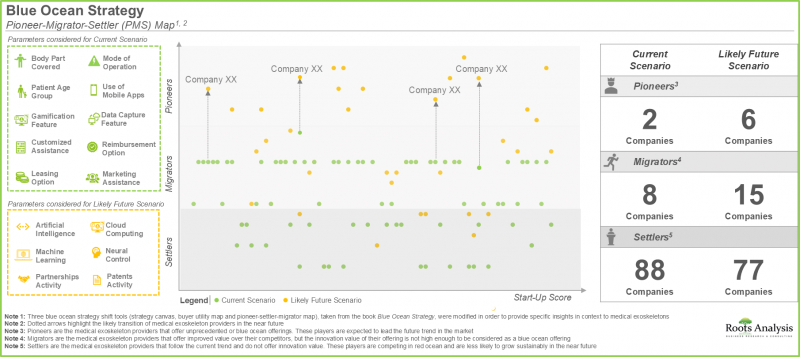
- 藍海策略分析中的 "先鋒-遷移者-先鋒" 模式表明,未來幾年,一些專注於提升產品功能的醫療外骨骼開發商有望成為行業先鋒。
- 預計到2035年,主要用於為肌肉無力和完全或部分截癱患者提供下肢支撐的剛性外骨骼的銷售額將佔外骨骼市場的約80%。
醫用外骨骼市場:主要細分市場
目前,下肢外骨骼細分市場在醫用外骨骼市場中佔最大佔有率。
根據目標身體部位,市場可細分為上肢外骨骼、下肢外骨骼及全身外骨骼。目前,下肢外骨骼細分市場在醫用外骨骼市場中佔最大佔有率。這主要是由於下肢外骨骼價格較高,以及提供下肢醫用外骨骼的公司數量不斷增加。 值得注意的是,全身醫用外骨骼市場預計將以更高的複合年增長率成長。
在預測期內,被動式外骨骼細分市場將是外骨骼市場中成長最快的細分市場。
依運作模式劃分,市場可分為動力外骨骼、被動式外骨骼及混合式外骨骼。預計被動式外骨骼細分市場在整個預測期內將以相對較快的速度成長。這是因為它們無需驅動器或電池即可提供必要的符合人體工學的支撐,從而降低了用戶的初始成本和維護成本。然而,目前動力醫用外骨骼細分市場佔了醫用外骨骼市場的最大佔有率。 這一趨勢在短期內不太可能改變。
目前,剛性外骨骼在醫用外骨骼市場中佔最大佔有率。
依類型劃分,市場分為剛性外骨骼和柔性外骨骼。由於其廣泛應用,剛性外骨骼在醫用外骨骼市場中佔主導地位。但值得注意的是,柔性外骨骼市場可望以相對較高的複合年增長率成長。
目前,移動式醫用外骨骼在醫用外骨骼市場中佔最大佔有率。
就移動性而言,市場分為固定式外骨骼和移動式外骨骼。值得注意的是,移動式/地面行走式醫用外骨骼目前在醫用外骨骼市場中佔更大的佔有率。 這是因為外骨骼能夠幫助患者參與恢復功能獨立性所需的活動,例如站立、爬樓梯和行走。此外,移動式外骨骼可在多種環境中使用,包括門診診所、家庭和公共場所,使患者能夠在傳統醫療機構之外繼續進行復健治療。
目前,面向患者的外骨骼預計將佔醫療外骨骼市場最大的佔有率。
依最終用戶劃分,市場分為患者和醫療保健專業人員。值得注意的是,面向患者的外骨骼佔了現有醫療外骨骼市場的大部分佔有率。
北美佔最大的市佔率。
依主要地區劃分,市場分為北美、歐洲、亞太地區及世界其他地區。值得注意的是,預計未來幾年世界其他地區的複合年增長率將更高。
外骨骼市場代表性公司
- Bionic Yantra
- CYBERDYNE
- Ekso Bionics
- ExoAtlet
- Fourier Intelligence
- Gloreha
- 廣州益康醫療器械
- Hexar Humancare
- Hocoma
- MediTouch
- Milebot Robotics
- Myomo
- Neofect
- NextStep Robotics
- Panasonic
- ReWalk Robotics
- Rex Bionics
- Roam Robotics
- Trexo Robotics
- Tyromotion
- U&O Technologies
主要研究概述
本研究中提出的觀點和見解是透過與多位利害關係人的討論而形成的。 本研究報告包含對以下行業專業人士的詳細訪談:
- A公司聯合創辦人兼首席執行官
- B公司業務規劃與發展總監
- C公司銷售與行銷副總裁
- D公司行銷與設計總監
- E公司創辦人兼董事
醫用外骨骼市場:研究範圍
- 市場規模與機會分析:本報告對醫用外骨骼市場進行了詳細分析,重點關注以下關鍵細分市場:[A] 目標身體部位,[B] 操作模式,[C] 移動性,[D] 最終用戶,以及 [F] 主要地區。
- 市場概況:本報告對醫用外骨骼設備進行了全面評估,內容涵蓋[A]研發現狀、[B]目標身體部位、[C]操作模式、[D]外骨骼形態、[E]設備移動性、[F]人機界面、[G]外骨骼高級功能、[H]最終用戶、[I]患者人口統計特徵、[JK]患者人口統計特徵、[JK]。本部分還包括以下資訊:[L]外骨骼技術/軟體、[M]外骨骼最大重量、[N]最大承重能力以及[O]外骨骼尺寸。 本章還列出了參與醫療外骨骼開發和商業化的主要公司,並根據以下指標重點介紹了最活躍的公司:[P] 成立年份,[Q] 公司規模,[R] 總部所在地,[S] 公司所有權,[T] 提供的附加服務,以及 [U] 提供的醫療外骨骼數量。
- 產品競爭力:對醫療外骨骼進行全面的競爭分析,具體包括:[A] 供應商優勢,[B] 產品競爭力,以及 [C] 最終用戶。我們將考慮以下因素:
- 公司簡介(詳細簡介):提供醫療外骨骼的主要公司的詳細簡介。重點關注:[A] 公司概況,[B] 財務資訊(如有),[C] 產品組合,[D] 近期發展,以及 [E] 未來展望。
- 公司簡介(表格):提供醫療外骨骼的主要公司的簡要概述。主要關注:[A] 公司概況和 [B] 產品組合。
- 合作夥伴關係:我們將根據以下參數分析自 2017 年以來該領域達成的合作夥伴關係:[A] 合作年份,[B] 合作類型(併購、產品開發和商業化協議、許可協議、服務協議、產品開發和製造協議、合資企業、製造和供應協議、產品分銷協議),[C] 合作夥伴(本節也涵蓋了該市場合作活動的區域分佈。
- 專利分析:基於[A]專利類型、[B]專利申請年份、[C]專利公開年份、[D]地理位置、[E]申請人類型、[F]公開時間、[G]主要CPC分類號和[H]主要公司(基於專利申請/授權數量)對各類專利申請/授權註冊進行詳細分析,並[I]提供詳細的專利基準分析。
- 藍海策略:基於藍海策略對當前和未來市場進行全面分析。為新興醫療外骨骼公司提供全面的策略計畫/指南,幫助其開拓未開發的市場。重點介紹了13種策略工具,可協助其轉型進入藍海市場,從而獲得市場競爭優勢。
- 市場影響分析:本報告分析影響市場成長的各種因素,包括驅動因素、限制因素、機會與挑戰。
目錄
第一章:引言
第二章:研究方法
第三章:經濟及其他專案特定考量
- 章節概述
- 市場動態
第四章:摘要整理
第五章:導論
- 章節概述
- 外骨骼概述
- 外骨骼發展史
- 外骨骼分類
- 外骨骼特性
- 外骨骼局限性
- 外骨骼應用
- 未來展望外骨骼
第六章 醫用外骨骼:市場概況
- 章節概述
- 醫用外骨骼:市場概況
- 醫用外骨骼:開發商概況
第七章 非醫用外骨骼:市場概況
- 章節概述
- 非醫用外骨骼:市場概況
- 非醫用外骨骼:開發商概況
第八章 醫用外骨骼:產品競爭分析
- 章節概述
- 假設和關鍵參數
- 研究方法
- 醫用外骨骼:產品競爭分析
第九章外骨骼開發商:公司簡介
- 章節概述
- CYBERDYNE
- Ekso Bionics
- ExoAtlet
- Fourier Intelligence
- Gloreha
- 廣州益康
- Hexar Humancare
- Hocoma
- Panasonic
- Tyromotion
第十章 外骨骼開發商:公司簡介
- 章節概述
- Bionic Yantra
- MediTouch
- Milebot Robotics
- Myomo
- Neofect
- NextStep機器人
- ReWalk Robotics
- Rex Bionics
- Roam Robotics
- Trexo Robotics
- U&O Technologies
第11章 醫療外骨骼:合作與夥伴關係
- 章節概述
- 合作模式
- 醫療外骨骼:合作與夥伴關係列表
第12章:專利分析
第13章:藍海戰略
- 藍海策略概述
第14章:市場影響分析:驅動因素、限制因素、機會與挑戰
章節第15章:全球外骨骼市場
第16章:依身體部位劃分的外骨骼市場
第17章:依運動模式劃分的外骨骼市場
第18章:依形態劃分的外骨骼市場
第19章:依性別劃分的外骨骼市場
第20章:依最終使用者劃分的外骨骼市場
第21章:按地區劃分的外骨骼市場
第22章:結論
第23章:高階主管洞察
第24章:附錄一:藍海策略與轉型工具
- 章節概述
- 藍海策略及轉型工具
- 藍海策略流程
第25章:附錄二:表格資料
第26章:附錄三:公司及組織列表
Exoskeleton Market: Overview
As per Roots Analysis, the global exoskeleton market is estimated to grow from USD 2.9 billion in the current year to USD 20.3 billion by 2035, at a CAGR of 22.4% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Body Part Covered:
- Upper Extremity
- Lower Extremity
- Full Body
Mode of Operation:
- Powered
- Passive
- Hybrid
Form:
- Powered
- Passive
- Hybrid
Mobility
- Fixed / Supported
- Mobile
End Users
- Patients
- Healthcare Providers
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Medical Exoskeleton Market: Growth and Trends
Over the years, significant advancements have occurred in robotic technologies, leading to enhanced usability of exoskeletons across different sectors for aiding and supporting humans. Especially in healthcare, these devices have demonstrated significant potential in aiding both patients and medical professionals. It is important to mention that even after decades of study and numerous possible applications, exoskeletons have not become the favored choice for stakeholders because of competition from other mobility systems and prosthetic devices often used in clinical and domestic environments.
Exoskeletons in healthcare mainly support rehabilitation, gait training, and mobility for those with spinal cord injuries (SCI), strokes, neurological conditions, or age-related disabilities. Further, demographic changes, especially in areas such as Asia-Pacific, heighten the demand for mobility improvements and elder care, with exoskeletons boosting rehabilitation results by 23% in motor function recovery at certain US clinics.
To ensure widespread approval, developers are concentrating on meeting real needs with their exoskeleton designs. They are actively seeking innovations to address issues related to cost-efficiency and user discomfort, striving to turn exoskeletons into more organic and integrated second skin solutions instead of cumbersome robotic suits. Additionally, developers are incorporating artificial intelligence and machine learning to allow devices to recognize the user's intentions and enhance human-robot interactions via suitable control algorithms. Moreover, innovations such as virtual reality (VR), augmented reality (AR), tailored games, or a mix of these methods are being implemented to improve robotic therapy by boosting patient involvement and ensuring they stay dedicated to completing their assigned exercises. Due to technological progress and continual developments in this area, exoskeleton devices are expected to have significant potential in the years to come.
Exoskeleton Market: Key Insights
The report delves into the current state of the exoskeleton market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Presently, over 200 exoskeletons are available / under development to provide support to patients with mobility impairments or reduce the risk of musculoskeletal injuries among caregivers dealing with disabled patients.

- Over 100 medical exoskeletons, both rigid and soft, cover different parts of the body and are typically operated using batteries that power the sensors and actuators; over 60% of them capture the motion metrics of patients.
- Developers of medical exoskeletons have established a global presence; more than 40% of these players are based in Europe, primarily in countries, such as Germany and Switzerland.
- With the growing demand for medical exoskeletons amongst patients, several types of strategic deals have been inked in this domain; majority (26%) of partnerships signed were distribution agreements.
- In order to increase adoption rates and maximize return on investment (ROI) for the users, developers of medical exoskeletons are striving to reduce costs while incorporating advanced features into their portfolio of devices.
- Nearly 3,800 patents related to exoskeletons have been filed / granted since 2016, indicating the substantial efforts made by researchers engaged in this domain.
- The pioneer-migrator-settler map in blue ocean strategy analysis suggests that several medical exoskeleton developers focused on enhancing their product features are likely to emerge as pioneers in the coming years.

- In 2035, nearly 80% of the exoskeleton market is likely to be driven by the sales of rigid exoskeletons primarily supporting the lower body, in patients with muscle weaknesses or complete / partial paraplegia.

Medical Exoskeleton Market: Key Segments
Currently, Lower Body Exoskeleton Segment Occupies the Largest Share of the Medical Exoskeleton Market
In terms of the body part covered, the market is segmented into upper body exoskeletons, lower body exoskeletons and full body exoskeletons. At present, the lower body exoskeleton segment holds the maximum share of the medical exoskeleton market. This is due to the steep price of lower body exoskeletons and an increase in the number of companies providing lower body medical exoskeletons. It is worth highlighting that the exoskeleton market for full body medical exoskeletons is anticipated to grow at a higher CAGR.
Passive Exoskeletons Segment is the Fastest Growing Segment of the Exoskeleton Market During the Forecast Period
In terms of the mode of operation, the market is segmented into powered exoskeletons, passive exoskeletons and hybrid exoskeletons. The passive exoskeletons segment is expected to expand at a comparatively faster growth rate throughout the forecast period. This is due to the devices offering necessary ergonomic assistance without requiring actuators or batteries, resulting in decreased initial costs and lower maintenance expenses for the users. However, currently, the powered medical exoskeleton segment holds the maximum share of the medical exoskeleton market. This trend is unlikely to change in the near future.
Currently, Rigid Exoskeletons Segment Occupies the Largest Share of the Medical Exoskeleton Market
In terms of their form, the market is segmented into rigid exoskeletons and soft exoskeletons. Due to their extensive use, rigid exoskeletons dominate the medical exoskeleton market. It is worth noting that soft exoskeletons segment is likely to grow at a relatively higher CAGR.
Currently, Mobile Medical Exoskeletons Occupy the Largest Share of the Medical Exoskeleton Market
In terms of mobility, the market is segmented into stationary exoskeletons and mobile exoskeletons. It is worth highlighting that, at present, mobile / overground walking medical exoskeleton holds a larger share of the medical exoskeleton market. This is owing to the fact that exoskeletons enable patients to engage in activities such as standing, climbing stairs, or walking, which are vital for restoring functional independence. Moreover, mobile exoskeletons can be utilized in different settings, such as outpatient clinics, residential areas, and communal spaces, allowing patients to pursue their rehabilitation beyond conventional healthcare environments.
Currently, Patient-Focused Exoskeletons are Expected to Capture the Largest Share of the Medical Exoskeleton Market
In terms of the end-user, the market is segmented into patients and healthcare professionals. It is important to note that a significant share of the existing medical exoskeleton market is dominated by patient-oriented exoskeletons.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the world. It is worth highlighting that, over the years, the market in the rest of the world is expected to grow at a higher CAGR.
Example Players in the Exoskeleton Market
- Bionic Yantra
- CYBERDYNE
- Ekso Bionics
- ExoAtlet
- Fourier Intelligence
- Gloreha
- Guangzhou Yikang Medical Equipment
- Hexar Humancare
- Hocoma
- MediTouch
- Milebot Robotics
- Myomo
- Neofect
- NextStep Robotics
- Panasonic
- ReWalk Robotics
- Rex Bionics
- Roam Robotics
- Trexo Robotics
- Tyromotion
- U&O Technologies
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Co-Founder and Chief Executive Officer, Company A
- Director of Business Planning and Development, Company B
- Vice President of Sales and Marketing, Company C
- Marketing and Design Manager, Company D
- Founder and Director, Company E
Medical Exoskeleton Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the exoskeleton market, focusing on key market segments, including [A] body part covered, [B] mode of operation, [C] mobility, [D] end users and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of medical exoskeletons, considering various parameters, such as [A] status of development, [B] body part covered, [C] mode of operation, [D] form of exoskeleton, [E] device mobility, [F] user-machine interface, [G] advanced features of exoskeleton, [H] end users, [I] patient age group, [J] exoskeleton setting for patients and [K] grant of breakthrough device designation. Additionally, it includes information on [L] exoskeleton technology / software, [M] maximum weight of exoskeleton, [N] maximum weight carrying capacity and [O] exoskeleton dimensions. Further, the chapter presents a list of players engaged in the development / commercialization of medical exoskeletons, along with information on their [P] year of establishment, [Q] company size, [R] location of headquarters, [S] company ownership, [T] additional services offered and [U] most active companies (in terms of number of medical exoskeleton offered).
- Product Competitiveness: A comprehensive competitive analysis of medical exoskeletons, examining factors, such as [A] supplier strength, [B] product competitiveness and [C] end users.
- Company Profiles (Detailed Profiles): In-depth profiles of key players offering medical exoskeletons, focusing on [A] company overviews, [B] financial information (if available), [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Company Profiles (Tabulated Profiles): Short profiles of key players offering medical exoskeletons, focusing on [A] company overviews and [B] product portfolio.
- Partnerships and Collaborations: An analysis of partnerships established in this sector, since 2017, based on several parameters, such as [A] year of partnership, [B] type of partnership (mergers and acquisitions, product development and commercialization agreements, licensing agreements, service agreements, product development and manufacturing agreements, joint ventures, manufacturing and supply agreements, and product distribution agreements), [C] type of partner (industry and non-industry), [D] business globalization and [E] most active players. This section also highlights the regional distribution of partnership activity in this market.
- Patent Analysis: Detailed analysis of various patents filed / granted based on [A] type of patent, [B] patent application year, [C] patent publication year, [D] geographical location, [E] type of applicant, [F] publication time, [G] top CPC symbols, [H] leading players (in terms of number of patents filed / granted), along with [I] a detailed patent benchmarking analysis.
- Blue Ocean Strategy: A comprehensive analysis of current and future market based on blue ocean strategy, covering a strategic plan / guide for emerging medical exoskeleton companies to help unlock an uncontested market, highlighting thirteen strategic tools that can help to shift towards blue ocean to gain a competitive edge in the market.
- Market Impact Analysis: The report analyzes various factors such as drivers, restraints, opportunities, and challenges affecting market growth.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Research Methodology
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Overview of Exoskeletons
- 5.3. History of Exoskeletons
- 5.4. Classification of Exoskeletons
- 5.4.1. Based on Body Part Supported
- 5.4.2. Based on Form of Exoskeleton
- 5.4.3. Based on Mode of Operation
- 5.4.4 Based on Mobility
- 5.5. Features of Exoskeletons
- 5.6. Limitations of Exoskeletons
- 5.7. Applications of Exoskeletons
- 5.8. Future Aspects of Exoskeletons
6. MEDICAL EXOSKELETONS: MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. Medical Exoskeletons: Overall Market Landscape
- 6.2.1. Analysis by Status of Development
- 6.2.2. Analysis by Type of Body Part Covered
- 6.2.3. Analysis by Mode of Operation
- 6.2.4. Analysis by Type of Body Part Covered and Mode of Operation
- 6.2.5. Analysis by Form of Exoskeleton
- 6.2.6. Analysis by Mode of Operation and Form of Exoskeleton
- 6.2.7. Analysis by Type of Body Part Covered and Form of Exoskeleton
- 6.2.8. Analysis by Device Mobility
- 6.2.9. Analysis by Mode of Operation and Device Mobility
- 6.2.10. Analysis by Form of Exoskeleton and Device Mobility
- 6.2.11. Analysis by Type of Body Part Covered and Device Mobility
- 6.2.12. Analysis by User-Machine Interface
- 6.2.13. Analysis by Type of Body Part Covered and User-Machine Interface
- 6.2.14. Analysis by Mode of Operation and User-Machine Interface
- 6.2.15. Analysis by Availability of Advanced Features
- 6.2.16. Analysis by End User
- 6.2.17. Analysis by Patient Age Group
- 6.2.18. Analysis by Exoskeleton Setting for Patients
- 6.2.19. Analysis by Breakthrough Designation
- 6.3. Medical Exoskeletons: Developer: Landscape
- 6.3.1. Analysis by Year of Establishment
- 6.3.2. Analysis by Company Size
- 6.3.3. Analysis by Location of Headquarters
- 6.3.4. Analysis by Company Size and Location of Headquarters (Region)
- 6.3.5. Analysis by Company Ownership
- 6.3.6. Analysis by Location of Headquarters (Region) and Company Ownership
- 6.3.7. Analysis by Additional Services Offered
- 6.3.8. Most Active Players: Analysis by Number of Medical Exoskeletons
7. NON-MEDICAL EXOSKELETONS: MARKET LANDSCAPE
- 7.1. Chapter Overview
- 7.2. Non-Medical Exoskeletons: Overall Market Landscape
- 7.2.1. Analysis by Status of Development
- 7.2.2. Analysis by Type of Body Part Covered
- 7.2.3. Analysis by Body Part Supported
- 7.2.4. Analysis by Mode of Operation
- 7.2.5. Analysis by Form of Exoskeleton
- 7.2.6. Analysis by Type of Body Part Covered and Mode of Operation
- 7.2.7. Analysis by Type of Body Part Covered and Form of Exoskeleton
- 7.2.8. Analysis by Mode of Operation and Form of Exoskeleton
- 7.2.9. Analysis by Application Area
- 7.2.10. Analysis by Mode of Operation and Application Area
- 7.3. Non-Medical Exoskeletons: Developer Landscape
- 7.3.1. Analysis by Year of Establishment
- 7.3.2. Analysis by Company Size
- 7.3.3. Analysis by Company Size and Employee Count
- 7.3.4. Analysis by Location of Headquarters
- 7.3.5. Analysis by Company Size and Location of Headquarters (Region)
- 7.3.6. Analysis by Company Ownership
- 7.3.7. Analysis by Location of Headquarters (Region) and Company Ownership
- 7.3.8. Most Active Players: Analysis by Number of Non-Medical Exoskeletons
- 7.3.9. Most Active Players: Analysis by Number of Exoskeletons
8. MEDICAL EXOSKELETONS: PRODUCT COMPETITIVENESS ANALYSIS
- 8.1. Chapter Overview
- 8.2. Assumptions and Key Parameters
- 8.3. Methodology
- 8.4. Medical Exoskeletons: Product Competitiveness Analysis
- 8.4.1. Product Competitiveness Analysis: Upper Body Medical Exoskeletons
- 8.4.1.1. Product Competitiveness Analysis: Powered Upper Body Exoskeletons
- 8.4.1.2. Product Competitiveness Analysis: Passive Upper Body Exoskeletons
- 8.4.1.3. Product Competitiveness Analysis: Hybrid Upper Body Exoskeletons
- 8.4.2. Product Competitiveness Analysis: Lower Body Exoskeletons
- 8.4.2.1. Product Competitiveness Analysis: Powered Lower Body Exoskeletons
- 8.4.2.2. Product Competitiveness Analysis: Passive Lower Body Exoskeletons
- 8.4.2.3. Product Competitiveness Analysis: Hybrid Lower Body Exoskeletons
- 8.4.3. Product Competitiveness Analysis: Full Body Medical Exoskeletons
- 8.4.1. Product Competitiveness Analysis: Upper Body Medical Exoskeletons
9. EXOSKELETON DEVELOPERS: DETAILED COMPANY PROFILES
- 9.1. Chapter Overview
- 9.2. CYBERDYNE
- 9.2.1. Company Overview
- 9.2.2. Financial Information
- 9.2.3. Product Portfolio
- 9.2.4 Recent Developments and Future Outlook
- 9.3. Ekso Bionics
- 9.3.1. Company Overview
- 9.3.2. Financial Information
- 9.3.3. Product Portfolio
- 9.3.4 Recent Developments and Future Outlook
- 9.4. ExoAtlet
- 9.4.1. Company Overview
- 9.4.2. Product Portfolio
- 9.4.3. Recent Developments and Future Outlook
- 9.5. Fourier Intelligence
- 9.5.1. Company Overview
- 9.5.2. Product Portfolio
- 9.5.3. Recent Developments and Future Outlook
- 9.6. Gloreha
- 9.6.1. Company Overview
- 9.6.2. Product Portfolio
- 9.6.3. Recent Developments and Future Outlook
- 9.7. Guangzhou Yikang
- 9.7.1. Company Overview
- 9.7.2. Product Portfolio
- 9.7.3. Recent Developments and Future Outlook
- 9.8. Hexar Humancare
- 9.8.1. Company Overview
- 9.8.2. Product Portfolio
- 9.8.3. Recent Developments and Future Outlook
- 9.9. Hocoma
- 9.9.1. Company Overview
- 9.9.2. Product Portfolio
- 9.9.3. Recent Developments and Future Outlook
- 9.10. Panasonic
- 9.10.1. Company Overview
- 9.10.2. Financial Information
- 9.10.3. Product Portfolio
- 9.10.4. Recent Developments and Future Outlook
- 9.11. Tyromotion
- 9.11.1. Company Overview
- 9.11.2. Product Portfolio
- 9.11.3. Recent Developments and Future Outlook
10. EXOSKELETON DEVELOPERS: TABULATED COMPANY PROFILES
- 10.1. Chapter Overview
- 10.2. Bionic Yantra
- 10.2.1. Company Overview
- 10.2.2. Product Portfolio
- 10.3. MediTouch
- 10.3.1. Company Overview
- 10.3.2. Product Portfolio
- 10.4. Milebot Robotics
- 10.4.1. Company Overview
- 10.4.2. Product Portfolio
- 10.5. Myomo
- 10.5.1. Company Overview
- 10.5.2. Product Portfolio
- 10.6. Neofect
- 10.6.1. Company Overview
- 10.6.2. Product Portfolio
- 10.7. NextStep Robotics
- 10.7.1. Company Overview
- 10.7.2. Product Portfolio
- 10.8. ReWalk Robotics
- 10.8.1. Company Overview
- 10.8.2. Product Portfolio
- 10.9. Rex Bionics
- 10.9.1. Company Overview
- 10.9.2. Product Portfolio
- 10.10. Roam Robotics
- 10.10.1. Company Overview
- 10.10.2. Product Portfolio
- 10.11. Trexo Robotics
- 10.11.1. Company Overview
- 10.11.2. Product Portfolio
- 10.12. U&O Technologies
- 10.12.1. Company Overview
- 10.12.2. Product Portfolio
11. MEDICAL EXOSKELETONS: PARTNERSHIPS AND COLLABORATIONS
- 11.1. Chapter Overview
- 11.2. Partnership Models
- 11.3. Medical Exoskeletons: List of Partnerships and Collaborations
- 11.3.1. Analysis by Year of Partnership
- 11.3.2. Analysis by Type of Partnership
- 11.3.3. Analysis by Year and Type of Partnership
- 11.3.4. Analysis by Purpose of Partnership
- 11.3.5. Analysis by Type and Purpose of Partnership
- 11.3.6. Analysis by Type of Partner
- 11.3.7. Analysis by Year of Partnership and Type of Partner
- 11.3.8. Analysis by Type of Non-Industry Players
- 11.3.9. Most Active Players: Distribution by Number of Partnerships
- 11.3.10. Analysis by Geography
- 11.3.10.1. Local and International Agreements
- 11.3.10.2. Intracontinental and Intercontinental Agreements
12. PATENT ANALYSIS
- 12.1. Chapter Overview
- 12.2. Scope and Methodology
- 12.3. Exoskeletons: Patent Analysis
- 12.3.1. Analysis by Patent Application Year
- 12.3.2. Analysis by Patent Publication Year
- 12.3.3. Analysis by Type of Patent and Patent Publication Year
- 12.3.4. Analysis by Publication Time
- 12.3.5. Analysis by Patent Jurisdiction
- 12.3.6. Analysis by CPC symbols
- 12.3.7. Analysis by Type of Applicant
- 12.3.8. Leading Industry Players: Analysis by Number of Patents
- 12.3.9. Leading Non-Industry Players: Analysis by Number of Patents
- 12.3.10. Leading Patent Assignees: Analysis by Number of Patents
- 12.4. Exoskeletons: Patent Benchmarking
- 12.4.1. Analysis by Patent Characteristics
- 12.4.2. Exoskeleton: Patent Valuation
- 12.5. Leading Players by Number of Citations
13. BLUE OCEAN STRATEGY
- 13.1. Overview of Blue Ocean Strategy
- 13.1.1. Red Oceans
- 13.1.2. Blue Oceans
- 13.1.3. Comparison of Red Ocean Strategy and Blue Ocean Strategy
- 13.1.4. Medical Exoskeletons: Blue Ocean Strategy and Shift Tools
- 13.1.4.1. Strategy Canvas
- 13.1.4.2. Pioneer-Migrator-Settler (PMS) Map
- 13.1.4.3. Buyer Utility Map
14. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 14.1. Chapter Overview
- 14.2. Market Drivers
- 14.3. Market Restraints
- 14.4. Market Opportunities
- 14.5. Market Challenges
- 14.6. Conclusion
15. GLOBAL EXOSKELETONS MARKET
- 15.1. Chapter Overview
- 15.2. Forecast Methodology and Key Assumptions
- 15.3. Global Exoskeletons Market, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 15.3.1. Scenario Analysis
- 15.3.1.1. Conservative Scenario
- 15.3.1.2. Optimistic Scenario
- 15.3.1. Scenario Analysis
- 15.4. Key Market Segmentations
16. EXOSKELETONS MARKET, BY BODY PART COVERED
- 16.1. Chapter Overview
- 16.2. Forecast Methodology and Key Assumptions
- 16.3. Overall Exoskeletons Market: Distribution by Body Part Covered
- 16.3.1. Overall Upper Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.3.2. Overall Lower Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.3.3. Overall Full Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.4. Medical Exoskeletons Market: Distribution by Body Part Covered
- 16.4.1 Medical Upper Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.4.2. Medical Lower Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.4.3. Medical Full Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.5. Non-Medical Exoskeletons Market: Distribution by Body Part Covered
- 16.5.1. Non-Medical Upper Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.5.2. Non-Medical Lower Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.5.3. Non-Medical Full Body Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 16.6. Data Triangulation and Validation
17. EXOSKELETONS MARKET, BY MODE OF OPERATION
- 17.1. Chapter Overview
- 17.2. Forecast Methodology and Key Assumptions
- 17.3. Overall Exoskeletons Market: Distribution by Mode of Operation
- 17.3.1. Overall Powered Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.3.2. Overall Passive Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.3.3. Overall Hybrid Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.4. Medical Exoskeletons Market: Distribution by Mode of Operation
- 17.4.1. Medical Powered Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.4.2. Medical Passive Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.4.3. Medical Hybrid Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.5. Non-Medical Exoskeletons Market: Distribution by Mode of Operation
- 17.5.1. Non-Medical Powered Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.5.2. Non-Medical Passive Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.5.3. Non-Medical Hybrid Exoskeletons Market: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 17.6. Data Triangulation and Validation
18. EXOSKELETON MARKETS, BY THEIR FORM
- 18.1. Chapter Overview
- 18.2. Forecast Methodology and Key Assumptions
- 18.3. Overall Exoskeletons Market: Distribution by Form
- 18.3.1. Overall Rigid Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 18.3.2. Overall Soft Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 18.4. Medical Exoskeletons Market: Distribution by Form
- 18.4.1. Medical Rigid Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 18.4.2. Medical Soft Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 18.5. Non-Medical Exoskeletons Market: Distribution by Form
- 18.5.1. Non-Medical Rigid Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 18.5.2. Non-Medical Soft Exoskeletons: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 18.6. Data Triangulation and Validation
19. EXOSKELETON MARKETS, BY THEIR MOBILITY
- 19.1. Chapter Overview
- 19.2. Forecast Methodology and Key Assumptions
- 19.3. Medical Exoskeletons Market: Distribution by Mobility
- 19.3.1. Medical Fixed/ Supported Exoskeleton: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 19.3.2. Medical Mobile / Overground Walking Exoskeleton: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 19.4. Data Triangulation and Validation
20. EXOSKELETONS MARKET, BY END USERS
- 20.1. Chapter Overview
- 20.2. Forecast Methodology and Key Assumptions
- 20.3. Overall Exoskeletons Market: Distribution by End Users
- 20.4. Exoskeletons Market for Patients: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 20.5. Exoskeletons Market for Healthcare Providers: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 20.6. Exoskeletons Market for Industry Workers: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 20.7. Exoskeletons Market for Military Personnel: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 20.8. Exoskeletons Market for Other End Users: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 20.9. Data Triangulation and Validation
21. EXOSKELETONS MARKET, BY GEOGRAPHY
- 21.1. Chapter Overview
- 21.2. Forecast Methodology and Key Assumptions
- 21.3. Overall Exoskeletons Market: Distribution by Geography
- 21.3.1. North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 21.3.2 Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 21.3.3. Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 21.3.4. Rest of the World, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 21.4. Data Triangulation and Validation
22. CONCLUSION
23. EXECUTIVE INSIGHTS
- 23.1. Chapter Overview
- 23.2. Company A
- 23.2.1. Company Snapshot
- 23.2.2. Interview Transcript: Co-Founder and Chief Executive Officer
- 23.3. Company B
- 23.3.1. Company Snapshot
- 23.3.2. Interview Transcript: Director of Business Planning and Development
- 23.4. Company C
- 23.4.1. Company Snapshot
- 23.4.2. Interview Transcript: Vice President of Sales and Marketing
- 23.5. Company D
- 23.5.1. Company Snapshot
- 23.5.2. Interview Transcript: Founder and Director
- 23.6. Company E
- 23.6.1. Company Snapshot
- 23.6.2. Interview Transcript: Marketing and Design Manager
24. APPENDIX I: BLUE OCEAN STRATEGY AND SHIFT TOOLS
- 24.1. Chapter Overview
- 24.2. Blue Ocean Strategy and Shift Tools
- 24.2.1. Value Innovation
- 24.2.2. Four Action Framework
- 24.2.3. Eliminate-Raise-Reduce-Create (ERRC) Grid
- 24.2.4. Six Path Framework
- 24.2.5. Three Tiers of Non-customers
- 24.3 Sequence of Blue Ocean Strategy
- 24.3.1. The Price Corridor of the Mass
- 24.3.2. Four Hurdles to Strategy Execution
- 24.3.3. Tipping Point Leadership
- 24.3.4. Fair Process
25. APPENDIX II: TABULATED DATA
26. APPENDIX III: LIST OF COMPANIES AND ORGANIZATION
List of Tables
- Table 5.1 Degrees of Freedom in Each Joint of Lower Exoskeletons
- Table 5.2 Contrasting Characteristics: Medical and Non-Medical Exoskeletons
- Table 5.3 Distinguishing Characteristics: Medical and Non-medical Exoskeletons
- Table 6.1 Medical Exoskeletons: Information on Status of Development, Type of Body Part Covered and Mode of Operation
- Table 6.2 Medical Exoskeletons: Information on Form, Device Mobility and User-Machine Interface
- Table 6.3 Medical Exoskeletons: Information on Advanced Features and End Users
- Table 6.4 Medical Exoskeletons: Information on Patient Age Group, Exoskeleton Setting for Patients and Breakthrough Designation
- Table 6.5 Medical Exoskeletons: Information on Technology / Software, Maximum Weight of Exoskeleton, Maximum Weight Carrying Capacity and Dimensions
- Table 6.6 Medical Exoskeletons: List of Developers
- Table 6.7 Medical Exoskeletons: Information on Additional Services Offered
- Table 7.1 Non-Medical Exoskeletons: Information on Status of Development, Type of Body Part Covered and Body Part Supported
- Table 7.2 Non-Medical Exoskeletons: Information on Mode of Operation and Form of Exoskeleton
- Table 7.3 Non-Medical Exoskeletons: Information on Application Area
- Table 7.4 Non-Medical Exoskeletons: Information on Technology / Software, Maximum Weight of Exoskeleton, Maximum Weight Carrying Capacity and Dimensions
- Table 7.5 Non-Medical Exoskeletons: List of Developers
- Table 9.1 List of Exoskeleton Companies Profiled
- Table 9.2 CYBERDYNE: Company Snapshot
- Table 9.3 CYBERDYNE: Medical Exoskeleton Portfolio
- Table 9.4 CYBERDYNE: Non-Medical Exoskeleton Portfolio
- Table 9.5 CYBERDYNE: Recent Developments and Future Outlook
- Table 9.6 Ekso Bionics: Company Snapshot
- Table 9.7 Ekso Bionics: Medical Exoskeleton Portfolio
- Table 9.8 Ekso Bionics: Non-Medical Exoskeleton Portfolio
- Table 9.9 Ekso Bionics: Recent Developments and Future Outlook
- Table 9.10 ExoAtlet: Company Snapshot
- Table 9.11 ExoAtlet: Medical Exoskeleton Portfolio
- Table 9.12 ExoAtlet: Non-Medical Exoskeleton Portfolio
- Table 9.13 ExoAtlet: Recent Developments and Future Outlook
- Table 9.14 Fourier Intelligence: Company Snapshot
- Table 9.15 Fourier Intelligence: Medical Exoskeleton Portfolio
- Table 9.16 Fourier Intelligence: Recent Developments and Future Outlook
- Table 9.17 Gloreha: Company Snapshot
- Table 9.18 Gloreha: Medical Exoskeleton Portfolio
- Table 9.19 Gloreha: Recent Developments and Future Outlook
- Table 9.20 Guangzhou YiKang: Company Snapshot
- Table 9.21 Guangzhou YiKang: Medical Exoskeleton Portfolio
- Table 9.22 Guangzhou YiKang: Recent Developments and Future Outlook
- Table 9.23 Hexar Humancare: Company Snapshot
- Table 9.24 Hexar Humancare: Medical Exoskeleton Portfolio
- Table 9.25 Hexar Humancare: Non-Medical Exoskeleton Portfolio
- Table 9.26 Hexar Humancare: Recent Developments and Future Outlook
- Table 9.27 Hocoma: Company Snapshot
- Table 9.28 Hocoma: Medical Exoskeleton Portfolio
- Table 9.29 Hocoma: Recent Developments and Future Outlook
- Table 9.30 Panasonic: Company Snapshot
- Table 9.31 Panasonic: Medical Exoskeleton Portfolio
- Table 9.32 Panasonic: Non-Medical Exoskeleton Portfolio
- Table 9.33 Panasonic: Recent Developments and Future Outlook
- Table 9.34 Tyromotion: Company Snapshot
- Table 9.35 Tyromotion: Medical Exoskeleton Portfolio
- Table 9.36 Tyromotion: Recent Developments and Future Outlook
- Table 10.1 Exoskeleton Developers: List of Companies Profiled
- Table 10.2 Bionic Yantra: Company Snapshot
- Table 10.3 Bionic Yantra: Medical Exoskeleton Portfolio
- Table 10.4 MediTouch: Company Snapshot
- Table 10.5 MediTouch: Medical Exoskeleton Portfolio
- Table 10.6 Milebot Robotics: Company Snapshot
- Table 10.7 Milebot Robotics: Medical Exoskeleton Portfolio
- Table 10.8 Milebot Robotics: Non- Medical Exoskeleton Portfolio
- Table 10.9 Myomo: Company Snapshot
- Table 10.10 Myomo: Medical Exoskeleton Portfolio
- Table 10.11 Neofect: Company Snapshot
- Table 10.12 Neofect: Medical Exoskeleton Portfolio
- Table 10.13 NextStep Robotics: Company Snapshot
- Table 10.14 NextStep Robotics: Medical Exoskeleton Portfolio
- Table 10.15 ReWalk Robotics: Company Snapshot
- Table 10.16 ReWalk Robotics: Medical Exoskeleton Portfolio
- Table 10.17 REX Bionics: Company Snapshot
- Table 10.18 REX Bionics: Medical Exoskeleton Portfolio
- Table 10.19 Roam Robotics: Company Snapshot
- Table 10.20 Roam Robotics: Medical Exoskeleton Portfolio
- Table 10.21 Roam Robotics: Non-Medical Exoskeleton Portfolio
- Table 10.22 Trexo Robotics: Company Snapshot
- Table 10.23 Trexo Robotics: Medical Exoskeleton Portfolio
- Table 10.24 U&O Technologies: Company Snapshot
- Table 10.25 U&O Technologies: Medical Exoskeleton Portfolio
- Table 11.1 Medical Exoskeletons: List of Partnerships and Collaborations, Since 2017
- Table 12.1 Patent Analysis: Top CPC Sections
- Table 12.2 Patent Analysis: Top CPC Symbols
- Table 12.3 Patent Analysis: Top CPC Codes
- Table 12.4 Patent Analysis: Summary of Benchmarking Analysis
- Table 12.5 Patent Analysis: Categorization based on Weighted Valuation Scores
- Table 12.6 Patent Portfolio: List of Leading Patents (by Highest Relative Valuation)
- Table 12.7 Patent Portfolio: List of Leading Patents (by Number of Citations)
- Table 15.1 Average Price of Powered Exoskeletons Used across Different Application Areas
- Table 16.1 Estimated Revenues of Medical Exoskeletons, based on Body Part Covered
- Table 16.2 Estimated Revenues of Non-Medical Exoskeletons, based on Body Part Covered
- Table 17.1 Estimated Revenues of Powered, Passive and Hybrid Exoskeletons from Various Application Areas
- Table 18.1 Estimated Revenues of Medical Exoskeletons, based on Form
- Table 18.2 Estimated Revenues of Non-Medical Exoskeletons, based on Form
- Table 19.1 Estimated Revenues of Medical Exoskeletons, based on Mobility
- Table 20.1 Estimated Revenues for Medical Exoskeletons from Various End Users
- Table 20.2 Estimated Revenues for Non-Medical Exoskeletons from Various End Users
- Table 23.1 ABLE Human Motion: Key Highlights
- Table 23.2 Archelis: Key Highlights
- Table 23.3 Biomotum: Key Highlights
- Table 23.4 Bionic Yantra: Key Highlights
- Table 23.5 Bionic Power: Key Highlights
- Table 25.1 Medical Exoskeletons: Distribution by Status of Development
- Table 25.2 Medical Exoskeletons: Distribution by Type of Body Part Covered
- Table 25.3 Medical Exoskeletons: Distribution by Mode of Operation
- Table 25.4 Medical Exoskeletons: Distribution by Type of Body Part Covered and Mode of Operation
- Table 25.5 Medical Exoskeletons: Distribution by Form of Exoskeleton
- Table 25.6 Medical Exoskeletons: Distribution by Mode of Operation and Form of Exoskeleton
- Table 25.7 Medical Exoskeletons: Distribution by Type of Body Part Covered and Form of Exoskeleton
- Table 25.8 Medical Exoskeletons: Distribution by Device Mobility
- Table 25.9 Medical Exoskeletons: Distribution by Mode of Operation and Device Mobility
- Table 25.10 Medical Exoskeletons: Distribution by Form of Exoskeleton and Device Mobility
- Table 25.11 Medical Exoskeletons: Distribution by Type of Body Part Covered and Device Mobility
- Table 25.12 Medical Exoskeletons: Distribution by User-Machine Interface
- Table 25.13 Medical Exoskeletons: Distribution by Type of Body Part Covered and User-Machine Interface
- Table 25.14 Medical Exoskeletons: Distribution by Mode of Operation and User-Machine Interface
- Table 25.15 Medical Exoskeletons: Distribution by Availability of Advanced Features
- Table 25.16 Medical Exoskeletons: Distribution by End User
- Table 25.17 Medical Exoskeletons: Distribution by Patient Age Group
- Table 25.18 Medical Exoskeletons: Distribution by Exoskeleton Setting for Patients
- Table 25.19 Medical Exoskeletons: Distribution by Breakthrough Designation
- Table 25.20 Medical Exoskeleton Developers: Distribution by Year of Establishment
- Table 25.21 Medical Exoskeleton Developers: Distribution by Company Size
- Table 25.22 Medical Exoskeleton Developers: Distribution by Company Size and Employee Count
- Table 25.23 Medical Exoskeleton Developers: Distribution by Location of Headquarters (Region)
- Table 25.24 Medical Exoskeleton Developers: Distribution by Location of Headquarters (Country)
- Table 25.25 Medical Exoskeleton Developers: Distribution by Company Size and Location of Headquarters
- Table 25.26 Medical Exoskeleton Developers: Distribution by Company Ownership
- Table 25.27 Medical Exoskeleton Developers: Distribution by Location of Headquarters and Company Ownership
- Table 25.28 Medical Exoskeleton Developers: Distribution by Additional Services Offered
- Table 25.29 Most Active Players: Distribution by Number of Medical Exoskeletons
- Table 25.30 Non-Medical Exoskeletons: Distribution by Status of Development
- Table 25.31 Non-Medical Exoskeletons: Distribution by Type of Body Part Covered
- Table 25.32 Non-Medical Exoskeletons: Distribution by Body Part Supported
- Table 25.33 Non-Medical Exoskeletons: Distribution by Mode of Operation
- Table 25.34 Non-Medical Exoskeletons: Distribution by Form of Exoskeleton
- Table 25.35 Non-Medical Exoskeletons: Distribution by Mode of Operation and Form of Exoskeleton
- Table 25.36 Non-Medical Exoskeletons: Distribution by Type of Body Part Covered and Mode of Operation
- Table 25.37 Non-Medical Exoskeletons: Distribution by Mode of Operation and Form of Exoskeleton
- Table 25.38 Non-Medical Exoskeletons: Distribution by Type of Body Part Covered and Form of Exoskeleton
- Table 25.39 Non-Medical Exoskeletons: Distribution by Application Area
- Table 25.40 Non-Medical Exoskeletons: Distribution by Mode of Operation and Application Area
- Table 25.41 Non-Medical Exoskeletons Developers: Distribution by Year of Establishment
- Table 25.42 Non-Medical Exoskeleton Developers: Distribution by Company Size
- Table 25.43 Non-Medical Exoskeleton Developers: Distribution by Company Size and Employee Count
- Table 25.44 Non-Medical Exoskeleton Developers: Distribution by Location of Headquarters (Region)
- Table 25.45 Non-Medical Exoskeleton Developers: Distribution by Location of Headquarters (Country)
- Table 25.46 Non-Medical Exoskeleton Developers: Distribution by Company Size and Location of Headquarters
- Table 25.47 Non-Medical Exoskeleton Developers: Distribution by Company Ownership
- Table 25.48 Non-Medical Exoskeleton Developers: Distribution by Location of Headquarters and Company Ownership
- Table 25.49 Most Active Players: Distribution by Number of Non-Medical Exoskeletons
- Table 25.50 Most Active Players: Distribution by Number of Medical and Non-Medical Exoskeletons
- Table 25.51 CYBERDYNE: Annual Revenues, Since FY2019 (JPY Billion)
- Table 25.52 CYBERDYNE: Distribution of Annual Revenues by Business Segment, Since FY 2021 (JPY Billion)
- Table 25.53 Ekso Bionics: Annual Revenues, Since 2018 (USD Billion)
- Table 25.54 Ekso Bionics: Distribution of Annual Revenues by Business Segment, FY 2023
- Table 25.55 Panasonic: Annual Revenues, FY 2018- FY 2023 (JPY Billion)
- Table 25.56 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2017
- Table 25.57 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 25.58 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Table 25.59 Partnerships and Collaborations: Distribution by Purpose of Partnership
- Table 25.60 Partnerships and Collaborations: Distribution by Type and Purpose of Partnership
- Table 25.61 Partnerships and Collaborations: Distribution by Type of Partner
- Table 25.62 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Table 25.63 Partnerships and Collaborations: Type of Non-Industry Partner
- Table 25.64 Most Active Players: Distribution by Number of Partnerships
- Table 25.65 Partnerships and Collaborations: Local and International Agreements
- Table 25.66 Partnerships and Collaborations: Intracontinental and Intercontinental Agreements
- Table 25.67 Patent Analysis: Distribution by Type of Patent
- Table 25.68 Patent Analysis: Cumulative Year-wise Trend by Patent Application Year, Since 2016
- Table 25.69 Patent Analysis: Cumulative Year-wise Trend by Patent Publication Year, Since 2016
- Table 25.70 Patent Analysis: Distribution by Type of Patent and Patent Publication Year, Since 2016
- Table 25.71 Patent Analysis: Distribution by Publication Time
- Table 25.72 Patent Analysis: Distribution by Patent Jurisdiction (Region)
- Table 25.73 Patent Analysis: Distribution by Patent Jurisdiction (Country)
- Table 25.74 Patent Analysis: Distribution by Type of Applicant
- Table 25.75 Leading Industry Players: Distribution by Number of Patents
- Table 25.76 Leading Non-Industry Players: Distribution by Number of Patents
- Table 25.77 Leading Individual Assignees: Distribution by Number of Patents
- Table 25.78 Leading Industry Players: Benchmarking by Patent Characteristics (CPC Codes)
- Table 25.79 Leading Non-Industry Players: Benchmarking by Patent Characteristics (CPC Codes)
- Table 25.80 Patent Analysis: Distribution by Patent Age
- Table 25.81 Patent Analysis: Patent Valuation
- Table 25.82 Global Exoskeletons Market, Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.83 Global Exoskeletons Market, Forecasted Estimates (Till 2035) Conservative Scenario (USD Billion)
- Table 25.84 Global Exoskeletons Market, Forecasted Estimates (Till 2035), Optimistic Scenario (USD Billion)
- Table 25.85 Medical Exoskeletons Market: Distribution by Body Part Covered (USD Billion)
- Table 25.86 Medical Upper Body Exoskeleton Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.87 Medical Lower Body Exoskeleton Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.88 Medical Full Body Exoskeleton Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.89 Non-Medical Upper Body Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.90 Non-Medical Lower Body Exoskeleton Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.91 Non-Medical Full Body Exoskeleton Market Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- Table 25.92 Overall Exoskeletons Market: Distribution by Body Part Covered (USD Billion)
- Table 25.93 Overall Upper Body Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.94 Overall Lower Body Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.95 Overall Full Body Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.96 Medical Exoskeletons Market: Distribution by Mode of Operation (USD Billion)
- Table 25.97 Medical Powered Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.98 Medical Passive Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.99 Medical Hybrid Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.100 Non-Medical Exoskeletons Market: Distribution by Mode of Operation (USD Billion)
- Table 25.101 Non-Medical Powered Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.102 Non-Medical Passive Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.103 Non-Medical Hybrid Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.104 Overall Powered Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.105 Overall Passive Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.106 Overall Hybrid Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.107 Medical Exoskeletons Market: Distribution by Form (USD Billion)
- Table 25.108 Medical Rigid Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.109 Medical Soft Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.110 Non-Medical Exoskeletons Market: Distribution by Form (USD Billion)
- Table 25.111 Non-Medical Rigid Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.112 Non-Medical Soft Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.113 Overall Exoskeletons Market: Distribution by Form (USD Billion)
- Table 25.114 Overall Rigid Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- Table 25.115 Overall Soft Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.116 Medical Fixed / Supported Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.117 Medical Mobile / Overground Walking Exoskeletons Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.118 Overall Exoskeletons Market: Distribution by End Users (USD Billion)
- Table 25.119 Medical Exoskeletons Market by Patients: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.120 Medical Exoskeletons Market by Healthcare Professionals: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.121 Non-Medical Exoskeletons Market by Industry Workers: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.122 Non-Medical Exoskeletons Market by Military Personnel: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.123 Non-Medical Exoskeletons Market by Other End Users: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.124 Exoskeletons Market in North America: Historical Trends (Since 2018) Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.125 Exoskeletons Market in Europe: Historical Trends (Since 2018) Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.126 Exoskeleton Market in Asia-Pacific: Historical Trends (Since 2018) Forecasted Estimates (Till 2035) (USD Billion)
- Table 25.127 Exoskeleton Market in Rest of the World: Historical Trends (Since 2018) Forecasted Estimates (Till 2035) (USD Billion)
List of Figures
- Figure 2.1 Research Methodology: Project Assumptions
- Figure 2.2 Research Methodology: Forecast Methodology
- Figure 2.3 Research Methodology: Robust Quality Control
- Figure 2.4 Research Methodology: Key Market Segmentations
- Figure 3.1 Lessons Learnt from Past Recessions
- Figure 4.1 Executive Summary: Medical Exoskeletons Market Landscape
- Figure 4.2 Executive Summary: Non-Medical Exoskeletons Market Landscape
- Figure 4.3 Executive Summary: Partnerships and Collaborations
- Figure 4.4 Executive Summary: Patent Analysis
- Figure 4.5 Executive Summary: Overall Market Forecast and Opportunity Analysis
- Figure 4.6 Executive Summary: Medical Exoskeletons Market Forecast and Opportunity Analysis
- Figure 4.7 Executive Summary: Non-Medical Exoskeletons Market Forecast and Opportunity Analysis
- Figure 5.1 Types of Mobility Assistive Devices
- Figure 5.2 Historical Events Related to Exoskeletons
- Figure 5.3 Classification of Exoskeletons
- Figure 5.4 Movements Supported by Lower Body, Upper Body and Full Body Exoskeletons
- Figure 5.5 Components of a Powered / Robotic Exoskeletons
- Figure 5.6 Features of Exoskeletons
- Figure 5.7 Limitations of Exoskeletons
- Figure 5.8 Applications of Exoskeleton
- Figure 6.1 Medical Exoskeletons: Distribution by Status of Development
- Figure 6.2 Medical Exoskeletons: Distribution by Type of Body Part Covered
- Figure 6.3 Medical Exoskeletons: Distribution by Mode of Operation
- Figure 6.4 Medical Exoskeletons: Distribution Type of Body Part Covered and Mode of Operation
- Figure 6.5 Medical Exoskeletons: Distribution by Form of Exoskeleton
- Figure 6.6 Medical Exoskeletons: Distribution by Mode of Operation and Form of Exoskeleton
- Figure 6.7 Medical Exoskeletons: Distribution Type of Body Part Covered and Form of Exoskeleton
- Figure 6.8 Medical Exoskeletons: Distribution by Device Mobility
- Figure 6.9 Medical Exoskeletons: Distribution by Mode of Operation and Device Mobility
- Figure 6.10 Medical Exoskeletons: Distribution by Form and Device Mobility
- Figure 6.11 Medical Exoskeletons: Distribution by Type of Body Part Covered and Device Mobility
- Figure 6.12 Medical Exoskeletons: Distribution by User-Machine Interface
- Figure 6.13 Medical Exoskeletons: Distribution by Type of Body Part Covered and User-Machine Interface
- Figure 6.14 Medical Exoskeletons: Distribution by Mode of Operation and User-Machine Interface
- Figure 6.15 Medical Exoskeletons: Distribution by Availability of Advanced Features
- Figure 6.16 Medical Exoskeletons: Distribution by End User
- Figure 6.17 Medical Exoskeletons: Distribution by Patient Age Group
- Figure 6.18 Medical Exoskeletons: Distribution by Exoskeleton Setting for Patients
- Figure 6.19 Medical Exoskeletons: Distribution by Breakthrough Designation
- Figure 6.20 Medical Exoskeleton Developers: Distribution by Year of Establishment
- Figure 6.21 Medical Exoskeleton Developers: Distribution by Company Size
- Figure 6.22 Medical Exoskeleton Developers: Distribution by Company Size and Employee Count
- Figure 6.23 Medical Exoskeleton Developers: Distribution by Location of Headquarters (Region)
- Figure 6.24 Medical Exoskeleton Developers: Distribution by Location of Headquarters (Country)
- Figure 6.25 Medical Exoskeleton Developers: Distribution by Company Size and Location of Headquarters
- Figure 6.26 Medical Exoskeleton Developers: Distribution by Company Ownership
- Figure 6.27 Medical Exoskeleton Developers: Distribution by Location of Headquarters and Company Ownership
- Figure 6.28 Medical Exoskeleton Developers: Distribution by Additional Services Offered
- Figure 6.29 Most Active Players: Distribution by Number of Medical Exoskeleton
- Figure 7.1 Non-Medical Exoskeletons: Distribution by Status of Development
- Figure 7.2 Non-Medical Exoskeletons: Distribution by Type of Body Part Covered
- Figure 7.3 Non-Medical Exoskeletons: Distribution by Body Part Supported
- Figure 7.4 Non-Medical Exoskeletons: Distribution by Mode of Operation
- Figure 7.5 Non-Medical Exoskeletons: Distribution by Form of Exoskeleton
- Figure 7.6 Non-Medical Exoskeletons: Distribution by Type of Body Part Covered and Mode of Operation
- Figure 7.7 Non-Medical Exoskeletons: Distribution by Type of Body Part Covered and Form of Exoskeleton
- Figure 7.8 Non-Medical Exoskeletons: Distribution by Mode of Operation and Form of Exoskeleton
- Figure 7.9 Non-Medical Exoskeletons: Distribution by Application Area
- Figure 7.10 Non-Medical Exoskeletons: Distribution by Mode of Operation and Application Area
- Figure 7.11 Non-Medical Exoskeleton Developers: Distribution by Year of Establishment
- Figure 7.12 Non-Medical Exoskeleton Developers: Distribution by Company Size
- Figure 7.13 Non-Medical Exoskeleton Developers: Distribution by Company Size and Employee Count
- Figure 7.14 Non-Medical Exoskeleton Developers: Distribution by Location of Headquarters (Region)
- Figure 7.15 Non-Medical Exoskeleton Developers: Distribution by Location of Headquarters (Country)
- Figure 7.16 Non-Medical Exoskeleton Developers: Distribution by Company Size and Location of Headquarters
- Figure 7.17 Non-Medical Exoskeleton Developers: Distribution by Company Ownership
- Figure 7.18 Non-Medical Exoskeleton Developers: Distribution by Location of Headquarters and Company Ownership
- Figure 7.19 Most Active Players: Distribution by Number of Exoskeletons
- Figure 7.20 Most Active Players: Distribution by Number of Medical and Non-Medical Exoskeletons
- Figure 8.1 Product Competitiveness Analysis: Powered Upper Body Exoskeletons (Peer Group I)
- Figure 8.2 Product Competitiveness Analysis: Passive Upper Body Exoskeletons (Peer Group II)
- Figure 8.3 Product Competitiveness Analysis: Hybrid Upper Body Exoskeletons (Peer Group III)
- Figure 8.4 Product Competitiveness Analysis: Powered Lower Body Exoskeletons (Peer Group IV)
- Figure 8.5 Product Competitiveness Analysis: Passive Lower Body Exoskeletons (Peer Group V)
- Figure 8.6 Product Competitiveness Analysis: Hybrid Lower Body Exoskeletons (Peer Group VI)
- Figure 8.7 Product Competitiveness Analysis: Full Body Exoskeleton (Peer Group VII)
- Figure 9.1 CYBERDYNE: Annual Revenues, Since FY 2019 (JPY Billion)
- Figure 9.2 CYBERDYNE: Distribution of Revenues by Business Segment, Since FY 2021
- Figure 9.3 Ekso Bionics: Annual Revenues, 2018 - Q1 2023 (USD Billion)
- Figure 9.4 Ekso Bionics: Distribution of Revenues by Business Segment, FY 2023
- Figure 9.5 Panasonic: Annual Revenues, Since FY 2018 (JPY Billion)
- Figure 11.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2017
- Figure 11.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 11.3 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Figure 11.4 Partnerships and Collaborations: Distribution by Purpose of Partnership
- Figure 11.5 Partnerships and Collaborations: Distribution by Type and Purpose of Partnership
- Figure 11.6 Partnerships and Collaborations: Distribution by Type of Partner
- Figure 11.7 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Figure 11.8 Partnerships and Collaborations: Distribution by Type of Non-Industry Partner
- Figure 11.9 Most Active Players: Distribution by Number of Partnerships
- Figure 11.10 Partnerships and Collaborations: Local and International Agreements
- Figure 11.11 Partnerships and Collaborations: Intracontinental and Intercontinental Agreements
- Figure 12.1 Patent Analysis: Distribution by Type of Patent
- Figure 12.2 Patent Analysis: Cumulative Year-wise Trend by Patent Application Year, Since Pre
- Figure 12.3 Patent Analysis: Cumulative Year-wise Trend by Patent Publication Year, Since 2016
- Figure 12.4 Patent Analysis: Distribution by Type of Patent and Patent Publication Year, Since 2016
- Figure 12.5 Patent Analysis: Distribution by Publication Time
- Figure 12.6 Patent Analysis: Distribution by Patent Jurisdiction (Region)
- Figure 12.7 Patent Analysis: Distribution by Patent Jurisdiction (Country)
- Figure 12.8 Patent Analysis: Distribution by CPC Symbols
- Figure 12.9 Patent Analysis: Distribution by Type of Applicant
- Figure 12.10 Leading Industry Players: Distribution by Number of Patents
- Figure 12.11 Leading Non-Industry Players: Distribution by Number of Patents
- Figure 12.12 Leading Individual Assignees: Distribution by Number of Patents
- Figure 12.13 Patent Benchmarking Analysis: Distribution of CPC Codes by Leading Industry Players
- Figure 12.14 Patent Benchmarking Analysis: Distribution of CPC Codes by Leading Non- Industry Players
- Figure 12.15 Patent Analysis: Distribution by Patent Age
- Figure 12.16 Patent Analysis: Patent Valuation
- Figure 13.1 Differences Between Red Ocean Strategy and Blue Ocean Strategy
- Figure 13.2 Blue Ocean Strategy: Strategy Canvas
- Figure 13.3 Blue Ocean Strategy: Pioneer-Migrator-Settler (PMS) Map
- Figure 13.4 Blue Ocean Strategy: Buyer Utility Map
- Figure 15.1 Standard Demand-Price Curve for Exoskeleton
- Figure 15.2 Global Exoskeletons Market, Historical Trends (Since 2018) and Forecasted Estimates (Till 2035) (USD Billion)
- Figure 15.3 Global Exoskeletons Market, Forecasted Estimates (Since 2023), Conservative Scenario (USD Billion)
- Figure 15.4 Global Exoskeletons Market, Forecasted Estimates (Since 2023), Optimistic Scenario (USD Billion)
- Figure 16.1 Overall Exoskeletons Market: Distribution by Body Part Covered (USD Billion)
- Figure 16.2 Overall Exoskeletons Market for Upper Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 16.3 Overall Exoskeletons Market for Lower Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (2023- 2035) (USD Billion)
- Figure 16.4 Overall Exoskeletons Market for Full Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 16.5 Medical Exoskeletons Market: Distribution by Body Part Covered, 2018, 2023 and 2035 (USD Billion)
- Figure 16.6 Medical Exoskeletons Market for Upper Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 16.7 Medical Exoskeletons Market for Lower Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 16.8 Medical Exoskeletons Market for Full Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 16.9 Non-Medical Exoskeletons Market: Distribution by Body Part Covered (USD Billion)
- Figure 16.10 Non-Medical Exoskeletons Market for Upper Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 16.11 Non-Medical Exoskeletons Market for Lower Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (2023- 2035) (USD Billion)
- Figure 16.12 Non-Medical Exoskeletons Market for Full Body Exoskeletons: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.1 Overall Exoskeletons Market: Distribution by Mode of Operation (USD Billion)
- Figure 17.2 Overall Exoskeletons Market for Powered Exoskeletons, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.3 Overall Exoskeletons Market for Passive Exoskeletons, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.4 Overall Exoskeletons Market for Hybrid Exoskeletons, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.5 Medical Exoskeletons Market: Distribution by Mode of Operation (USD Billion)
- Figure 17.6 Medical Powered Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.7 Medical Passive Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.8 Medical Hybrid Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.9 Non-Medical Exoskeletons Market: Distribution by Mode of Operation (USD Billion)
- Figure 17.10 Non-Medical Powered Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.11 Non-Medical Passive Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 17.12 Non-Medical Hybrid Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 18.1 Overall Exoskeletons Market: Distribution by Form (USD Billion)
- Figure 18.2 Overall Exoskeletons Market for Rigid Exoskeletons, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 18.3 Overall Exoskeletons Market for Soft Exoskeletons, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 18.4 Medical Exoskeletons Market: Distribution by Form (USD Billion)
- Figure 18.5 Medical Rigid Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 18.6 Medical Soft Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 18.7 Non-Medical Exoskeletons Market: Distribution by Form (USD Billion)
- Figure 18.8 Non-Medical Rigid Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 18.9 Non-Medical Soft Exoskeletons Market, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 19.1 Medical Exoskeletons Market: Distribution by Mobility (USD Billion)
- Figure 19.2 Medical Fixed / Supported Exoskeletons Market: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 19.3 Medical Mobile / Overground Walking Exoskeletons Market: Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 20.1 Overall Exoskeletons Market: Distribution by End Users (USD Billion)
- Figure 20.2 Exoskeletons Market for Patients, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 20.3 Exoskeletons Market for Healthcare Professionals, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 20.4 Exoskeletons Market for Industry Workers, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 20.5 Exoskeletons Market for Military Personnel, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 20.6 Exoskeletons Market for Other End Users, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 21.1 Overall Exoskeletons Market: Distribution by Geography
- Figure 21.2 Exoskeletons Market in North America, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 21.3 Exoskeletons Market in Europe, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 21.4 Exoskeletons Market in Asia-Pacific, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 21.5 Exoskeletons Market in Rest of the World, Historical Trends (Since 2018) and Future Estimates (Till 2035) (USD Billion)
- Figure 22.1 Concluding Remarks: Overall Medical Exoskeletons Market Landscape
- Figure 22.2 Concluding Remarks: Overall Non-Medical Exoskeletons Market Landscape
- Figure 22.3 Concluding Remarks: Partnerships and Collaborations
- Figure 22.4 Concluding Remarks: Patent Analysis
- Figure 22.5 Concluding Remarks: Market Forecast and Opportunity Analysis (I/II)
- Figure 22.6 Concluding Remarks: Market Forecast and Opportunity Analysis (II/II)
- Figure 24.1 Blue Ocean Strategy: Three Tiers of Non-customers
- Figure 24.2 Blue Ocean Strategy: Sequence of Blue Ocean Strategy
- Figure 24.3 Blue Ocean Strategy: The Price Corridor of The Mass








