 |
市場調查報告書
商品編碼
1891247
臨床試驗中心支持機構市場:產業趨勢及全球預測(至 2035 年)-按治療領域、試驗階段、臨床試驗組成部分、介入類型和主要地區劃分Site Management Organization Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Therapeutic Area, Trial Phases, Clinical Trial Components, Type of Interventions and Key Geographies |
||||||
臨床試驗中心支持機構市場:市場概覽
據估計,臨床試驗中心支援機構市場規模將從今年的 124 億美元增長至 2035 年的 316 億美元,預測期內(至 2035 年)的複合年增長率 (CAGR) 為 9.8%。
臨床試驗中心支持機構市場:成長與趨勢
臨床試驗是整個藥物研發過程中至關重要的階段,在評估候選藥物的安全性和有效性方面發揮著不可或缺的作用。研究表明,用於候選藥物研發的總投資中約有 40% 用於臨床試驗,每年的成本高達 780 億美元。 然而,進行這些試驗往往面臨諸多挑戰,包括科學和操作上的複雜性、招募和留住合適患者的挑戰、數據管理方面的挑戰以及嚴格的監管標準。
此外,整個流程固有的複雜性以及多方利害關係人的參與,使得這些試驗容易延誤。超過 80% 的臨床試驗會延誤 1 至 6 個月,而只有 10% 的試驗能夠準時完成。因此,製藥創新者不斷努力改善臨床試驗的進行和有效的管理方法。在眾多選擇中,將各種試驗職能外包給諸如臨床試驗中心管理機構 (SMO) 等專業服務提供者已成為許多研發者的熱門選擇。由於註冊臨床試驗的數量和複雜性不斷增加,預計在整個預測期內,對 SMO 的需求將穩定成長。
市場成長驅動因素:
臨床試驗中心管理機構 (SMO) 市場的成長動力源自於臨床試驗數量和複雜性的不斷增加。這是因為 II/III 期試驗和精準醫療的入組研究需要專業的現場支援。 此外,慢性病和罕見病患疾病率的不斷上升,推動了對利用電子健康記錄 (EHR) 和區域網路進行病患招募和留存的臨床試驗管理組織 (SMO) 專業知識的需求。向分散式和混合式試驗的轉變,推動了 SMO 在遠端監查、數位資源和數據準確性方面的應用。此外,外包趨勢、亞太和拉丁美洲新興市場的成長,以及人工智慧和電子知情同意書 (eConsent) 等技術的整合,正在進一步推動 SMO 的發展。對真實世界數據 (RWE) 和上市後監測日益增長的需求,正將 SMO 轉變為可擴展的高附加價值網路。
市場限制因素:
臨床試驗管理組織 (SMO) 市場面臨著許多限制因素,包括員工培訓、法規遵循和技術整合等高昂的營運成本,這使得 SMO 難以製定具有競爭力的價格。來自內部臨床試驗網絡、旗下擁有 SMO 的大型合約研究組織 (CRO) 以及綜合研究組織 (IRO) 的激烈競爭,正在阻礙 SMO 的市場佔有率增長,迫使規模較小的 SMO 專注於特定領域。 病患招募和留存仍然是一大挑戰,儘管臨床試驗支援組織 (SMO) 已盡力,但由於入組人數不足,導致試驗嚴重延誤。此外,對合約研究組織 (CRO) 與申辦者之間合約的依賴以及新興市場的經濟波動,使得 SMO 的收入難以預測,阻礙了市場擴張。
臨床試驗支持組織 (SMO) 市場:主要發現
本報告深入分析了目前臨床試驗支持組織市場的現狀,並指出了該行業的潛在成長機會。主要發現包括:
1. 目前,全球約有 250 家業者聲稱可為治療產品和醫療器材的臨床試驗申辦方提供臨床試驗支援服務。
2. 市場較為分散,既有老牌企業,也有新進入者,它們都聲稱能夠從地理位置分散的地點,為各種治療領域提供臨床試驗中心管理服務。
3. 該領域的公司正在穩步提升自身能力,以拓展服務組合併適應不斷變化的行業標準。
4. 在過去五年中,已簽署超過200項協議,其中大部分合作集中在服務合作和臨床試驗協議方面,旨在為申辦方公司提供臨床研究服務。
5. 眾多投資人看好未來的獲利前景,已向29家臨床試驗中心支持服務公司投資總計10億美元。
6. 2020年,全球註冊的臨床試驗約有10,000項,旨在評估各種潛在幹預措施,並開發循證醫學和醫療保健解決方案。
7. 隨著臨床試驗數量的持續增長,對研究參與者的需求也急劇上升,促使藥物研發公司轉向第三方服務提供者。
8. 目前超過60%的臨床試驗營運外包,預計未來十年該市場將保持穩定成長。
9. 預計市場機會可能分佈在各個治療領域和主要地理區域。
臨床試驗中心管理組織 (SMO) 市場
市場規模與機會分析依下列參數細分:
治療領域
- 腫瘤學
- 中樞神經系統疾病
- 傳染病
- 心血管疾病
- 其他
試驗階段
- I期
- II期
- III期
- IV期
臨床試驗組成
- 現場管理
- 現場監查
- 專案管理
- 其他
介入類型
- 治療方法
- 醫療器材
- 外科手術
主要地區
- 北美
- 歐洲
- 世界其他地區
臨床試驗中心管理機構 (SMO) 市場:主要細分市場
預計 SMO 市場將主要由旨在治療和管理腫瘤適應症的臨床試驗推動
依治療領域劃分,SMO 市場可細分為腫瘤、中樞神經系統、傳染病、呼吸系統、心血管系統、內分泌系統、胃腸道、肌肉骨骼系統、免疫系統、皮膚及其他。預計到 2035 年,SMO 市場將佔 38% 的市場佔有率,其中旨在治療和管理腫瘤適應症的臨床試驗將發揮主導作用。這主要是由於目前全球範圍內正在進行或計劃進行的臨床試驗數量不斷增加,這些試驗旨在研究針對腫瘤適應症的藥物的潛力。
預計在預測期內,II期臨床試驗的SMO市場將保持領先地位。
依臨床試驗階段劃分,整體市場分為I期、II期、III期及IV期。今年II期臨床試驗預計的SMO市場將佔市場佔有率的約40%,並在預測期內以12.1%的複合年增長率成長。
預計在預測期內,臨床試驗現場管理活動將引領臨床試驗現場支持組織市場。
就臨床試驗營運而言,整體市場分為現場管理、資料管理、品質控制、本地生產、法規事務、專案管理、物流和其他。預計今年現場管理活動(包括試驗中心選擇、合約簽訂和付款、試驗中心啟動和激活以及試驗中心關閉)的SMO市場將佔市場佔有率的約30%,並在預測期內以9.8%的複合年增長率增長。
預計在預測期內,治療藥物(藥品和生物製品)市場將以 9.8% 的複合年增長率成長。
就治療藥物而言,整體市場細分為外科手術、醫療器材和治療藥物。預計到 2035 年,治療藥物(藥品和生物製品)SMO 市場規模將達到 220 億美元,在預測期內以 9.8% 的複合年增長率成長。
預計北美將推動臨床試驗現場支持組織市場的成長。
按地區劃分,整體市場細分為北美、歐洲、亞太地區、拉丁美洲、中東和北非 (MENA) 以及世界其他地區 (RoW)。
今年,北美佔了整體臨床試驗現場支援市場的大部分佔有率,預計這一趨勢在可預見的未來將保持不變。 然而,亞太地區預計將呈現更快的成長,在預測期內複合年增長率將達到 13.7%。
臨床試驗現場支援機構市場最新動態:
臨床試驗現場支援機構領域近期出現了一些新的發展動態。以下列舉了一些近期措施。雖然這些發展動態發生在我們的市場報告發布之後,但它們與我們分析中概述的整體市場趨勢相符。
- Psyence Biomed 與澳洲臨床試驗網絡 (ACTioN) 簽署了策略合作協議,共同進行 IIb 期臨床試驗。
- Flourish Research 獲得了 Genstar Capital 的策略性投資,以進一步拓展其臨床研究服務。
- Neutra Corporation 收購了總部位於美國的臨床試驗現場支援機構 Mercury Clinical Research。此外,資產管理公司 Blackstone 宣布計劃收購總部位於東京的臨床試驗現場支援機構 I'rom Group。
臨床試驗機構支持組織 (CRO) 市場代表性公司
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS Health Science
- Trialbee
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
目錄
第一章:前言
第二章:摘要整理
第三章:導論
- 章節概要
- 臨床試驗中心支持機構
- 臨床試驗中心支援機構在臨床試驗中的作用
- 臨床試驗中心支援機構提供的服務
- 一站式服務的優勢
- 結論
第四章:競爭格局
- 章節概要
- 臨床試驗中心支援機構:市場格局
第五章:競爭分析
- 章節概要
- 假設和關鍵參數
- 研究方法
- 臨床試驗中心支援機構:競爭分析
第六章 公司簡介:北美臨床試驗中心支持機構
- 章節概述
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
第七章:公司簡介:歐洲臨床試驗中心支持機構
- 章節概述
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS健康科學
- Trialbee
第八章:公司簡介:亞太地區臨床試驗中心支持機構
- 章節概述
- CMIC集團
- George Clinical
- Tigermed
- Veeda Clinical Research
第九章:合作關係
- 章節概述
- 合作模式
- 臨床試驗中心支持機構:合作關係
第十章:資金與投資分析
- 章節概述
- 資金類型
- 臨床試驗中心支援機構:資金與投資分析
第 11 章:臨床試驗分析的主要的洞察
- 章節概述
- 研究範圍與方法
- 臨床試驗中心支援機構:臨床試驗的主要的洞察
第 12 章:臨床試驗參與者需求分析
- 章節概述
- 研究方法與關鍵假設
- 全球臨床試驗參與者需求:按入組患者族群分析
第 13 章:市場預測與機會分析
- 章節概述
- 預測方法與關鍵假設
- 2035 年全球臨床試驗中心支援機構市場
- 2021 年及以後依治療領域劃分的臨床試驗中心支持機構市場2035 年
- 依試驗階段劃分的臨床試驗現場管理機構市場,2035 年
- 依臨床試驗組成部分劃分的臨床試驗現場管理機構市場,2035 年
- 按幹預類型劃分的臨床試驗現場管理機構市場,2035 年
- 按地區劃分的臨床試驗現場管理機構市場,2021 年和 2035 年
第 14 章:結論
第 15 章:高階主管洞察
第 16 章,附錄 1:表格資料
第 17 章,附錄 2:公司與機構清單
SITE MANAGEMENT ORGANIZATION MARKET: OVERVIEW
As per Roots Analysis, the site management organization market is estimated to grow from USD 12.4 billion in the current year to USD 31. 6 billion by 2035, at a CAGR of 9.8% during the forecast period, till 2035.
SITE MANAGEMENT ORGANIZATIONS MARKET: GROWTH AND TRENDS
Clinical trials are a crucial step of the entire drug development process, allowing for the essential assessment of a drug candidate's safety and effectiveness. Research indicates that approximately 40% of the overall investment allocated for the advancement of drug candidates is spent on clinical trials, amounting to an annual cost of USD 78 billion. Nonetheless, conducting such trials frequently presents significant difficulties, including scientific and operational intricacies, challenges with recruitment and retention of appropriate patients, challenges associated with data management, and rigorous regulatory standards.
Additionally, due to the intrinsic complexity of the entire process and the participation of multiple stakeholders, these trials are susceptible to delays. Over 80% of clinical trials experience delays ranging from one to six months, whereas merely 10% of the studies finish on schedule. Consequently, innovators within the pharmaceutical sector are consistently working on enhancing methods for executing clinical trials and managing them effectively. Among other options, delegating different trial operations to a dedicated service provider, such as site management organizations (SMOs), has become a prominent choice for many developers. As the complexity increases and the number of registered clinical trials rises, the need for SMOs is expected to see consistent market growth throughout the forecast period.
Market Growth Drivers:
The site management organization (SMO) market is driven by increasing clinical trial volume and complexity, as registered studies require specialized site assistance for Phase II / III and precision medicine. Moreover, the increasing prevalence of chronic and rare diseases drives the need for SMOs' expertise in patient recruitment and retention via EHRs and community networks. The transition to decentralized and hybrid trials boosts SMO implementation for overseeing remote monitoring, digital resources, and data accuracy. Further, trends in outsourcing, growth of emerging markets in Asia-Pacific and LATAM, along with technology integration such as AI and eConsent propel further expansion. It is worth highlighting that the need for real-world evidence and post-marketing research is transforming SMOs into scalable, high-value networks.
Market Restraints:
The Site Management Organization (SMO) market faces considerable limitations due to high operational expenses, such as employee training, adherence to regulations, and technology integration which present competitive pricing challenges. Severe rivalry from internal site networks, major CROs with incorporated SMOs, and rising integrated research organizations (IROs) hampers market share and compels smaller SMOs to focus on niche sectors. Recruitment and retention of patients continue to pose challenges, leading to considerable trial delays from insufficient enrollment, even with SMO initiatives. Ultimately, reliance on CRO-sponsor agreements and economic fluctuations in emerging markets subject SMOs to revenue unpredictability, thus hindering their market expansion.
SITE MANAGEMENT ORGANIZATIONS MARKET: KEY INSIGHTS
The report delves into the current state of the site management organizations market and identifies potential growth opportunities within industry. Some key findings from the report include:
1. Presently, around 250 players across the globe claim to offer clinical trial site management services to trial sponsors, for both therapeutic products and medical devices.
2. The market is fragmented, featuring the presence of both established players and new entrants based in different geographies that claim to be capable of offering site management services, for wide range of therapeutic areas.

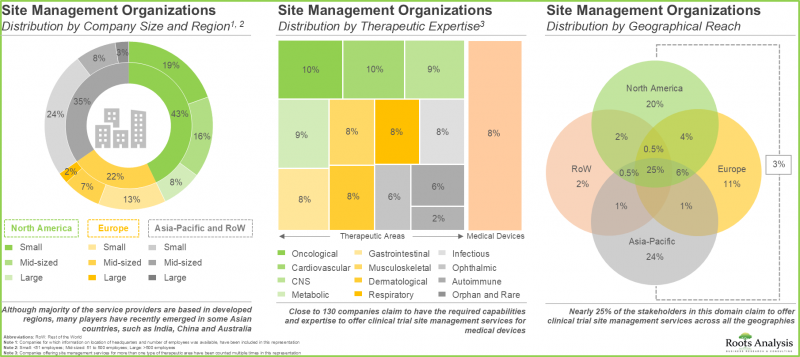
3. Companies involved in this domain are steadily expanding their capabilities in order to enhance their respective service portfolios and comply to evolving industry benchmarks.
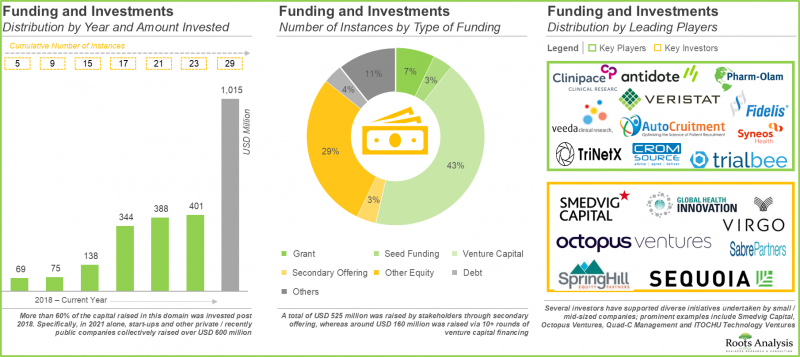
4. Over 200 deals have been inked in the past five years; majority of the reported collaborations were focused on forming service alliances and clinical trial agreements to offer clinical research services to sponsors.
5. Foreseeing a lucrative future, a large number of investors have invested capital worth USD 1 billion, across 29 instances, in companies offering clinical trial site management services.

6. In 2020, around 10,000 trials were registered to evaluate various types of potential interventions and develop evidence-based medicine and health care solutions, worldwide.
7. There has been a surge in the demand for study participants owing to the continuous growth in number of clinical trials; this has prompted drug developers to leverage services to third party service providers.
8. With more than 60% of the clinical trial operations currently being outsourced, we expect the market to grow at a steady pace over the next decade.

9. The anticipated opportunity is expected to be segregated across a variety of therapeutic areas; it is also likely to be well-distributed across key geographical regions.
Site Management Organization Market
The market sizing and opportunity analysis has been segmented across the following parameters:
Therapeutic Areas
- Oncological Disorders
- CNS Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
Trial Phases
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trial Components
- Site Management
- Onsite monitoring
- Project Management
- Others
Type of Interventions
- Therapeutics
- Devices
- Surgical Procedures
Key Geographies
- North America
- Europe
- Rest of the World
SITE MANAGEMENT ORGANIZATIONS MARKET: KEY SEGMENTS
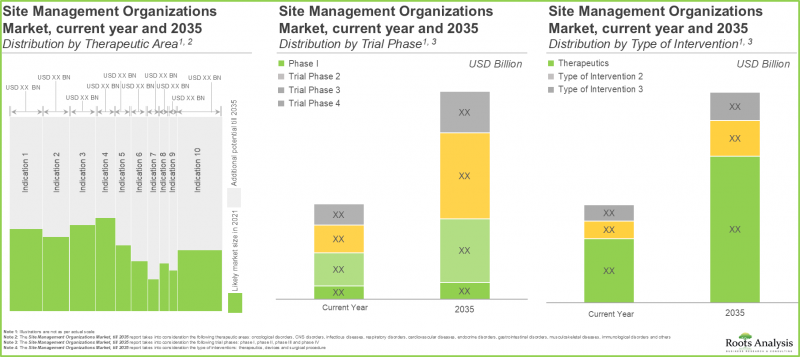
SMOs Market is Likely to be Dominated by Trial Studies Intended for the Treatment / Management of Oncological Indications
In terms of therapeutic area, the market is segmented across oncological disorders, CNS disorders, infectious diseases, respiratory disorders, cardiovascular diseases, endocrine disorders, gastrointestinal disorders, musculoskeletal diseases, immunological disorders, dermatological disorders and others. The SMOs market is likely to be dominated by trial studies intended for the treatment / management of oncological indications, capturing 38% of the market share by 2035. This is owing to an increase in number of trials currently being / anticipated to be conducted to investigate the potential of drugs targeting oncological indications, worldwide.
SMOs market for Phase II Trial Studies is Likely to Dominate during the Forecast Period
In terms of the trial phase, the overall market is segmented across Phase I, Phase II, Phase III and Phase IV. The SMOs market for phase II trial studies is likely to capture nearly 40% of the market share in the current year, growing at a CAGR of 12.1%, during the given time period
Site Management Activities is Anticipated to Lead the Site Management Organization Market during the Forecast Period
In terms of clinical trial operations, the overall market is segmented across site management, data management, quality control, onsite manufacturing, regulatory affairs, project management, logistics and others. The SMOs market for site management activities (including site identification and selection, site contracting and payments, site initiation and activation and site close-out) is likely to capture around 30% of the market share in the current year, growing at a CAGR of 9.8%, during the given forecast period.
Market For Therapeutics (Drugs and Biologics) is Likely to Grow at a CAGR of 9.8%, During the Given Time Period
In terms of therapeutics, the overall market is segmented across surgical procedures, devices and therapeutics. the SMOs market for therapeutics (drugs and biologics) is likely to be worth USD 22 billion in 2035, growing at a CAGR of 9.8%, during the given time period.
North America is Likely to Propel the Growth of the Site Management Organization Market
In terms of geographical regions, the overall market is segmented across North America, Europe, Asia-Pacific, Latin America, MENA and RoW.
In the current year, North America captures the majority share of the overall clinical trial site management market and this trend is unlikely to change in the foreseen future. However, Asia-Pacific is likely to grow at a faster growth rate, with a CAGR of 13.7% during the forecast period.
Recent Developments in Site Management Organization Market:
Several recent developments have taken place in the field of site management organization. We have outlined some of these recent initiatives below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that have been outlined in our analysis.
- Psyence Biomed entered into a strategic agreement with Australian Clinical Trial Network (ACTioN) for its Phase IIb clinical trial.
- Flourish Research received strategic investment from Genstar Capital to further expand its clinical research services.
- Neutra Corporation acquired Mercury Clinical Research, a US based site management organization. Blackstone, an asset management company, also announced its plans to acquire Tokyo based SMO I'rom Group.
Primary Research Overview
The opinions and insights presented in the market report were also influenced by discussions held with senior stakeholders in the industry. The market research report features detailed transcripts of interviews held with the following individuals:
- Country Head - Clinical Operations, Mid-sized Company, India
- Medical Director and Operations Manager, Small Company, Argentina
- Project Manager, Small Company, India
Example Players in Site Management Organizations Market
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS HealthScience
- Trialbee
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
SITE MANAGEMENT ORGANIZATIONS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the site management organizations market, focusing on key market segments, including [A] therapeutic area, [B] trial phases, [C] clinical trial components, [D] type of interventions, and [E] key geographical regions.
- Market Landscape: A detailed assessment of overall competitive landscape companies offering clinical trial management services to various organizations, including CROs, and pharmaceutical, biotechnology and medical devices companies based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] type of service offered, and [E] therapeutic expertise of service providers.
- Company Competitiveness Analysis: A comprehensive competitive analysis of service providers segregated into three peer groups based on location of their headquarters (North America, Europe, and Asia-Pacific and RoW), examining factors, such as [A] supplier strength [B] product strength and [C] application areas.
- Company Profiles: In-depth profiles of prominent players that offer various SMO services focusing on [A] year of establishment, [B] location of headquarters, [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Partnerships and Collaborations: An analysis of the partnerships that have been inked by stakeholders engaged in site management organization market, based on various parameters, such as [A] year of partnership, [B] type of partnership, [C] focus area and [D] most active players.
- Funding and Investment Analysis: A detailed analysis of various investments received by players engaged in site management organization market based on several relevant parameters, such as [A] year of investment, [B] number of funding instances, [C] amount invested, [D] type of funding (grant, seed, venture capital, secondary offering, other equity, debt and others) and [E] type of investor, [F] most active players, [G] most active investors and [H] geographical distribution (in terms of number of funding instances and amount invested).
- Clinical Trial Analysis: An in-depth analysis of completed, ongoing and planned clinical studies based on several relevant parameters, such as [A] trial registration year, [B] number of enrolled patients, [C] trial status, [D] trial phase, [E] type of sponsor and [F] geographical distribution of number of trials and enrolled patient population.
- Demand Analysis: An analysis of the annual demand for clinical study participants, taking into account the target patient population in ongoing and planned clinical trials, sponsored by both industry and non-industry players.
KEY QUESTIONS ANSWERED IN THIS REPORT
- What is a site-specific management organization and how does it operate?
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- Which region dominates the site management organizations market?
- What are the key trends observed in the site management organizations market?
- What factors are likely to influence the evolution of this market?
- What are the primary challenges faced by site management organizations?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Site Management Organizations
- 3.3. Role of Site Management Organizations in Clinical Trials
- 3.4. Services Offered by Site Management Organizations
- 3.5. Advantages of One-Stop-Shops
- 3.6. Concluding Remarks
4. COMPETITIVE LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Site Management Organizations: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Service(s) Offered
- 4.2.5. Analysis by Therapeutic Expertise
- 4.2.6. Analysis by Geographical Reach
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Site Management Organizations: Company Competitiveness Analysis
- 5.4.1. Competitiveness Analysis: Site Management Organizations in North America
- 5.4.1.1. Competitiveness Analysis: Small Companies in North America
- 5.4.1.2. Competitiveness Analysis: Mid-sized Companies in North America
- 5.4.1.3. Competitiveness Analysis: Large Companies in North America
- 5.4.2. Competitiveness Analysis: Site Management Organizations in Europe
- 5.4.2.1. Competitiveness Analysis: Small Companies in Europe
- 5.4.2.2. Competitiveness Analysis: Mid-sized Companies in Europe
- 5.4.2.3. Competitiveness Analysis: Large Companies in Europe
- 5.4.3. Competitiveness Analysis: Site Management Organizations in Asia-Pacific and Rest of the World
- 5.4.3.1. Competitiveness Analysis: Small Companies in Asia-Pacific and Rest of the World
- 5.4.3.2. Competitiveness Analysis: Mid-sized Companies in Asia-Pacific and Rest of the World
- 5.4.3.3. Competitiveness Analysis: Large Companies in Asia-Pacific and Rest of the World
- 5.4.1. Competitiveness Analysis: Site Management Organizations in North America
6. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN NORTH AMERICA
- 6.1. Chapter Overview
- 6.2. FOMAT Medical Research
- 6.2.1. Company Overview
- 6.2.2. Clinical Trial Site Management Services
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Parexel
- 6.3.1. Company Overview
- 6.3.2. Clinical Trial Site Management Services
- 6.3.3. Recent Developments and Future Outlook
- 6.4. Pharm-Olam
- 6.4.1. Company Overview
- 6.4.2. Clinical Trial Site Management Services
- 6.4.3. Recent Developments and Future Outlook
- 6.5. Veristat
- 6.5.1. Company Overview
- 6.5.2. Clinical Trial Site Management Services
- 6.5.3. Recent Developments and Future Outlook
- 6.6. WCCT Global
- 6.6.1. Company Overview
- 6.6.2. Clinical Trial Site Management Services
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Worldwide Clinical Trials
- 6.7.1. Company Overview
- 6.7.2. Clinical Trial Site Management Services
- 6.7.3. Recent Developments and Future Outlook
7. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN EUROPE
- 7.1. Chapter Overview
- 7.2. CROMSOURCE
- 7.2.1. Company Overview
- 7.2.2. Clinical Trial Site Management Services
- 7.2.3. Recent Developments and Future Outlook
- 7.3. Fidelis Research
- 7.3.1. Company Overview
- 7.3.2. Clinical Trial Site Management Services
- 7.3.3. Recent Developments and Future Outlook
- 7.4. Scandinavian CRO
- 7.4.1. Company Overview
- 7.4.2. Clinical Trial Site Management Services
- 7.4.3. Recent Developments and Future Outlook
- 7.5. TFS HealthScience
- 7.5.1. Company Overview
- 7.5.2. Clinical Trial Site Management Services
- 7.5.3. Recent Developments and Future Outlook
- 7.6. Trialbee
- 7.6.1. Company Overview
- 7.6.2. Clinical Trial Site Management Services
- 7.6.3. Recent Developments and Future Outlook
8. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN ASIA-PACIFIC
- 8.1. Chapter Overview
- 8.2. CMIC Group
- 8.2.1. Company Overview
- 8.2.2. Clinical Trial Site Management Services
- 8.2.3. Recent Developments and Future Outlook
- 8.3. George Clinical
- 8.3.1. Company Overview
- 8.3.2. Clinical Trial Site Management Service
- 8.3.3. Recent Developments and Future Outlook
- 8.4. Tigermed
- 8.4.1. Company Overview
- 8.4.2. Clinical Trial Site Management Services
- 8.4.3. Recent Developments and Future Outlook
- 8.5. Veeda Clinical Research
- 8.5.1. Company Overview
- 8.5.2. Clinical Trial Site Management Services
- 8.5.3. Recent Developments and Future Outlook
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Site Management Organizations: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Year and Type of Partnership
- 9.3.4. Analysis by Focus Area
- 9.3.5. Most Active Players: Analysis by Number of Partnerships
- 9.3.6. Geographical Analysis
- 9.3.6.1. Region-wise Distribution
- 9.3.6.2. Country-wise Distribution
10. FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding
- 10.3. Site Management Organizations: Funding and Investment Analysis
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.3.4. Year-wise Analysis by Type of Funding and Amount Invested
- 10.3.5. Most Active Players: Analysis by Number of Funding Instances
- 10.3.6. Most Active Investors: Analysis by Number of Funding Instances
- 10.3.7. Analysis by Type of Investor
- 10.3.8. Analysis by Geography
11. KEY INSIGHTS FROM CLINICAL TRIAL ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Site Management Organizations: Clinical Trial Key Insights
- 11.3.1. Analysis by Trial Registration Year
- 11.3.2. Analysis by Trial Registration Year and Enrolled Patient Population
- 11.3.3. Analysis by Trial Status
- 11.3.4. Analysis by Trial Registration Year and Trial Status
- 11.3.5. Analysis by Trial Phase
- 11.3.6. Analysis by Trial Phase and Enrolled Patient Population
- 11.3.7. Analysis by Trial Registration Year and Trial Phase (in terms of Number of Clinical Trials)
- 11.3.8. Analysis by Trial Registration Year and Trial Phase (in terms of Number of Enrolled Patient Population)
- 11.3.9. Analysis by Type of Sponsor / Collaborator
- 11.3.10. Geographical Analysis by Number of Clinical Trials
- 11.3.11. Geographical Analysis by Enrolled Patient Population
12. ANALYSIS OF DEMAND FOR CLINICAL TRIAL PARTICIPANTS
- 12.1. Chapter Overview
- 12.2. Methodology and Key Assumptions
- 12.3. Global Demand for Clinical Trial Participants: Analysis by Enrolled Patient Population
- 12.3.1. Analysis of Demand by Trial Phase
- 12.3.2. Analysis of Demand by Therapeutic Area
- 12.3.3. Geographical Demand by Enrolled Patient Population
13. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 13.1. Chapter Overview
- 13.2. Forecast Methodology and Key Assumptions
- 13.3. Global Site Management Organizations Market, Till 2035
- 13.4. Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area
- 13.5. Site Management Organizations Market, Till 2035: Distribution by Trial Phase
- 13.6. Site Management Organizations Market, Till 2035: Distribution by Clinical Trial Components
- 13.7. Site Management Organizations Market, Till 2035: Distribution by Type of Intervention
- 13.8. Site Management Organizations Market, 2021 and 2035: Distribution by Region
- 13.8.1. Site Management Organizations Market, 2021 and 2035: Distribution by Country
- 13.8.2. Site Management Organizations Market in North America, Till 2035
- 13.8.2.1. Site Management Organizations Market in the US, Till 2035
- 13.8.2.2. Site Management Organizations Market in Canada, Till 2035
- 13.8.2.3. Site Management Organizations Market in Rest of North America, Till 2035
- 13.8.3. Site Management Organizations Market in Europe, Till 2035
- 13.8.3.1. Site Management Organizations Market in the UK, Till 2035
- 13.8.3.2. Site Management Organizations Market in France, Till 2035
- 13.8.3.3. Site Management Organizations Market in Germany, Till 2035
- 13.8.3.4. Site Management Organizations Market in Spain, Till 2035
- 13.8.3.5. Site Management Organizations Market in Italy, Till 2035
- 13.8.3.6. Site Management Organizations Market in Rest of Europe, Till 2035
- 13.8.4. Site Management Organizations Market in Asia-Pacific, Till 2035
- 13.8.4.1. Site Management Organizations Market in China, Till 2035
- 13.8.4.2. Site Management Organizations Market in Korea, Till 2035
- 13.8.4.3. Site Management Organizations Market in India, Till 2035
- 13.8.4.4. Site Management Organizations Market in Australia, Till 2035
- 13.8.4.5. Site Management Organizations Market in Japan, Till 2035
- 13.8.4.6. Site Management Organizations Market in Israel, Till 2035
- 13.8.4.7. Site Management Organizations Market in Rest of Asia-Pacific, Till 2035
- 13.8.5. Site Management Organizations Market in Latin America, Till 2035
- 13.8.6. Site Management Organizations Market in MENA, Till 2035
- 13.8.7. Site Management Organizations Market in Rest of the World, Till 2035
14. CONCLUDING REMARKS
15. EXECUTIVE INSIGHTS
- 15.1. Chapter Overview
- 15.2. Company A
- 15.2.1. Company Snapshot
- 15.2.2. Interview Transcript: Country Head-Clinical Operations
- 15.3. Company B
- 15.3.1. Company Snapshot
- 15.3.2. Interview Transcript: Medical Director and Operations Manager
- 15.4. Company C
- 15.4.1. Company Snapshot
- 15.4.2. Interview Transcript: Project Manager
16. APPENDIX 1: TABULATED DATA
17. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Comparison of SMOs and CROs
- Table 4.1 List of Site Management Organizations
- Table 4.2 Site Management Organizations: Information on Type of Site Management Service(s) offered
- Table 4.3 Site Management Organizations: Information on Therapeutic Expertise
- Table 6.1 Site Management Organizations: List of Profiled Companies (North America)
- Table 6.2 FOMAT Medical Research: Company Snapshot
- Table 6.3 FOMAT Medical Research: Clinical Trial Site Management Service Portfolio
- Table 6.4 FOMAT Medical Research: Recent Developments and Future Outlook
- Table 6.5 Parexel: Company Snapshot
- Table 6.6 Parexel: Clinical Trial Site Management Service Portfolio
- Table 6.7 Paraxel: Recent Developments and Future Outlook
- Table 6.8 Pharm-Olam: Company Snapshot
- Table 6.9 Pharm-Olam: Clinical Trial Site Management Service Portfolio
- Table 6.10 Pharm-Olam: Recent Developments and Future Outlook
- Table 6.11 Veristat: Company Snapshot
- Table 6.12 Veristat: Clinical Trial Site Management Service Portfolio
- Table 6.13 Veristat: Recent Developments and Future Outlook
- Table 6.14 WCCT Global: Company Snapshot
- Table 6.15 WCCT Global: Clinical Trial Site Management Service Portfolio
- Table 6.16 WCCT Global: Recent Developments and Future Outlook
- Table 7.1 Site Management Organizations: List of Profiled Companies (Europe)
- Table 7.2 CROMSOURCE: Company Snapshot
- Table 7.3 CROMSOURCE: Clinical Trial Site Management Service Portfolio
- Table 7.4 CROMSOURCE: Recent Developments and Future Outlook
- Table 7.5 FIDELIS RESEARCH: Company Snapshot
- Table 7.6 FIDELIS RESEARCH: Clinical Trial Site Management Service Portfolio
- Table 7.7 FIDELIS RESEARCH: Recent Developments and Future Outlook
- Table 7.8 Scandinavian CRO: Company Snapshot
- Table 7.9 Scandinavian CRO: Clinical Trial Site Management Service Portfolio
- Table 7.10 Scandinavian CRO: Recent Developments and Future Outlook
- Table 7.11 TFS HealthScience: Company Snapshot
- Table 7.12 TFS HealthScience: Clinical Trial Site Management Service Portfolio
- Table 7.13 TFS HealthScience: Recent Developments and Future Outlook
- Table 7.14 Trialbee: Company Snapshot
- Table 7.15 Trialbee: Clinical Trial Site Management Service Portfolio
- Table 7.16 Trialbee: Recent Developments and Future Outlook
- Table 8.1 Site Management Organizations: List of Profiled Companies (Asia-Pacific)
- Table 8.2 CMIC Group: Company Snapshot
- Table 8.3 CMIC Group: Clinical Trial Site Management Service Portfolio
- Table 8.4 CMIC Group: Recent Developments and Future Outlook
- Table 8.5 George Clinical: Company Snapshot
- Table 8.6 George Clinical: Clinical Trial Site Management Service Portfolio
- Table 8.7 George Clinical: Recent Developments and Future Outlook
- Table 8.8 Tigermed: Company Snapshot
- Table 8.9 Tigermed: Clinical Trial Site Management Service Portfolio
- Table 8.10 Tigermed: Recent Developments and Future Outlook
- Table 8.11 Veeda Clinical Research: Company Snapshot
- Table 8.12 Veeda Clinical Research: Clinical Trial Site Management Service Portfolio
- Table 8.13 Veeda Clinical Research: Recent Developments and Future Outlook
- Table 9.1 Site Management Organizations: List of Partnerships and Collaborations, Since 2016
- Table 10.1 Site Management Organizations: List of Funding and Investments, Since 2015
- Table 12.1 Global Demand for Clinical Trial Participants: Average Number of Patients Enrolled by Trial Phase
- Table 16.1 Site Management Organizations: Distribution by Year of Establishment
- Table 16.2 Site Management Organizations: Distribution by Company Size
- Table 16.3 Site Management Organizations: Distribution by Location of Headquarters (Region-wise)
- Table 16.4 Site Management Organizations: Distribution by Location of Headquarters (Country-wise)
- Table 16.5 Site Management Organizations: Distribution by Company Size and Location of Headquarters
- Table 16.6 Site Management Organizations: Distribution by Service(s) Offered
- Table 16.7 Site Management Organizations: Distribution by Location of Headquarters and Service(s) Offered
- Table 16.8 Site Management Organizations: Distribution by Therapeutic Expertise
- Table 16.9 Site Management Organizations: Distribution by Geographical Reach
- Table 16.10 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Table 16.11 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 16.12 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Table 16.13 Partnerships and Collaborations: Distribution by Type of Partnership, Since 2016
- Table 16.14 Partnerships and Collaborations: Distribution by Focus Area
- Table 16.15 Most Active Players: Distribution by Number of Partnerships
- Table 16.16 Partnerships and Collaborations: Distribution by Region
- Table 16.17 Partnerships and Collaborations: Distribution by Country
- Table 16.18 Funding and Investment Analysis: Cumulative Year-wise Trend, Since 2015
- Table 16.19 Funding and Investment Analysis: Cumulative Year-wise Trend by Amount Invested, Since 2015 (USD Million)
- Table 16.20 Funding and Investment Analysis: Distribution by Type of Funding
- Table 16.21 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding (USD Million)
- Table 16.22 Funding and Investment Analysis: Year-wise Distribution by Amount Invested and Type of Funding, Since 2015 (USD Million)
- Table 16.23 Most Active Players: Distribution by Number of Funding Instances
- Table 16.24 Most Active Investors: Distribution by Number of Funding Instances
- Table 16.25 Funding and Investment Analysis: Distribution by Type of Investor
- Table 16.26 Funding and Investment Analysis: Distribution of Amount Invested by Type of Investor (USD Million)
- Table 16.27 Funding and Investment Analysis: Distribution by Region (Continent-wise)
- Table 16.28 Funding and Investment Analysis: Distribution by Region (Country-wise)
- Table 10.29 Funding and Investment Analysis: Distribution of Amount Invested by Region (Country-wise) (USD Million)
- Table 16.30 Clinical Trial Key Insights: Cumulative Distribution by Trial Registration Year, Since 2016
- Table 16.31 Clinical Trial Key Insights: Distribution by Trial Registration Year and Enrolled Patient Population, Since 2016
- Table 16.32 Clinical Trial Key Insights: Distribution by Trial Status
- Table 16.33 Clinical Trial Key Insights: Distribution by Trial Registration Year and Trial Status, Since 2016
- Table 16.34 Clinical Trial Key Insights: Distribution of Number of Trials by Trial Phase
- Table 16.35 Clinical Trial Key Insights: Distribution of Enrolled Patient Population by Trial Phase
- Table 16.36 Clinical Trial Key Insights: Year-wise Distribution by Number of Trials and Trial Phase, Since 2016
- Table 16.37 Clinical Trial Key Insights: Year-wise Distribution by Number of Enrolled Patient Population and Trial Phase, Since 2016
- Table 16.38 Clinical Trial Key Insights: Distribution by Type of Sponsor / Collaborator
- Table 16.39 Clinical Trial Key Insights: Geographical Distribution by Number of Clinical Trials
- Table 16.40 Clinical Trial Key Insights: Geographical Distribution by Enrolled Patient Population
- Table 16.41 Global Demand for Clinical Trial Participants, Till 2035 (Million Patients)
- Table 16.42 Global Demand for Clinical Trial Participants: Distribution by Therapeutic Area (Million Patients)
- Table 16.43 Global Demand for Clinical Trial Participants: Distribution by Trial Phase (Million Patients)
- Table 16.44 Global Demand for Clinical Trial Participants: Distribution by Region (Million Patients)
- Table 16.45 Global Site Management Organizations Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.46 Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area (USD Billion)
- Table 16.47 Site Management Organizations Market, Till 2035: Distribution by Trial Phase (USD Billion)
- Table 16.48 Site Management Organizations Market, 2021: Distribution by Clinical Trial Components (USD Billion)
- Table 16.49 Site Management Organizations Market, Till 2035: Distribution by Type of Intervention (USD Billion)
- Table 16.50 Site Management Organizations Market, 2021 and 2035: Distribution by Region (USD Billion)
- Table 16.51 Site Management Organizations Market, 2021 and 2035: Distribution by Country (USD Billion)
- Table 16.52 Site Management Organizations Market in North America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.53 Site Management Organizations Market in the US, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.54 Site Management Organizations Market in Canada, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.55 Site Management Organizations Market in Rest of North America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.56 Site Management Organizations Market in Europe, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.57 Site Management Organizations Market in the UK, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.58 Site Management Organizations Market in France, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.59 Site Management Organizations Market in Germany, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.60 Site Management Organizations Market in Spain, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.61 Site Management Organizations Market in Italy, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.62 Site Management Organizations Market in Rest of Europe, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.63 Site Management Organizations Market in Asia-Pacific, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.64 Site Management Organizations Market in China, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.65 Site Management Organizations Market in Korea, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.66 Site Management Organizations Market in India, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.67 Site Management Organizations Market in Australia, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.68 Site Management Organizations Market in Japan, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.69 Site Management Organizations Market in Israel, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.70 Site Management Organizations Market in Rest of Asia-Pacific, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.71 Site Management Organizations Market in Latin America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.72 Site Management Organizations Market in MENA, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.73 Site Management Organizations Market in Rest of the World, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
List of Figures
- Figure 2.1 Executive Summary: Market Forecast
- Figure 2.2 Executive Summary: Overall Market Landscape
- Figure 2.3 Executive Summary: Partnerships and Collaborations
- Figure 2.4 Executive Summary: Funding and Investments
- Figure 2.5 Executive Summary: Clinical Trial Key Insights
- Figure 2.6 Executive Summary: Analysis of Demand for Clinical Trial Participants
- Figure 3.1 Working Model of Site Management Organizations
- Figure 3.2 Services Offered by Site Management Organizations
- Figure 4.1 Site Management Organizations: Distribution by Year of Establishment
- Figure 4.2 Site Management Organizations: Distribution by Company Size
- Figure 4.3 Site Management Organizations: Distribution by Location of Headquarters (Region-wise)
- Figure 4.4 Site Management Organizations: Distribution by Location of Headquarters (Country-wise)
- Figure 4.5 Site Management Organizations: Distribution by Company Size and Location of Headquarters
- Figure 4.6 Site Management Organizations: Distribution by Service(s) Offered
- Figure 4.7 Site Management Organizations: Distribution by Location of Headquarters and Service(s) Offered
- Figure 4.8 Site Management Organizations: Distribution by Therapeutic Expertise
- Figure 4.9 Site Management Organizations: Distribution by Geographical Reach
- Figure 5.1 Company Competitiveness Analysis: Small Players in North America
- Figure 5.2 Company Competitiveness Analysis: Mid-sized Players in North America
- Figure 5.3 Company Competitiveness Analysis: Large Players in North America
- Figure 5.4 Company Competitiveness Analysis: Small Players in Europe
- Figure 5.5 Company Competitiveness Analysis: Mid-sized Players in Europe
- Figure 5.6 Company Competitiveness Analysis: Large Players in Europe
- Figure 5.7 Company Competitiveness Analysis: Small Players in Asia-Pacific and Rest of the World
- Figure 5.8 Company Competitiveness Analysis: Mid-sized Players in Asia-Pacific and Rest of the World
- Figure 5.9 Company Competitiveness Analysis: Large Players in Asia-Pacific and Rest of the World
- Figure 9.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Figure 9.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 9.3 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Figure 9.4 Partnerships and Collaborations: Distribution by Type of Partnership, Since 2016
- Figure 9.5 Partnerships and Collaborations: Distribution by Focus Area
- Figure 9.6 Most Active Players: Distribution by Number of Partnerships
- Figure 9.7 Partnerships and Collaborations: Distribution by Region
- Figure 9.8 Partnerships and Collaborations: Distribution by Country
- Figure 10.1 Funding and Investment Analysis: Cumulative Year-wise Trend, Since 2015
- Figure 10.2 Funding and Investment Analysis: Cumulative Year-wise Trend by Amount Invested, Since 2015 (USD Million)
- Figure 10.3 Funding and Investment Analysis: Distribution by Type of Funding
- Figure 10.4 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding (USD Million)
- Figure 10.5 Funding and Investment Analysis: Year-wise Distribution by Amount Invested and Type of Funding, Since 2015 (USD Million)
- Figure 10.6 Most Active Players: Distribution by Number of Funding Instances
- Figure 10.7 Most Active Investors: Distribution by Number of Funding Instances
- Figure 10.8 Funding and Investment Analysis: Distribution by Type of Investor
- Figure 10.9 Funding and Investment Analysis: Distribution of Amount Invested by Type of Investor (USD Million)
- Figure 10.10 Funding and Investment Analysis: Distribution by Region (Continent-wise)
- Figure 10.11 Funding and Investment Analysis: Distribution by Region (Country-wise)
- Figure 10.12 Funding and Investment Analysis: Distribution of Amount Invested by Region (Country-wise) (USD Million)
- Figure 11.1 Clinical Trial Key Insights: Scope and Methodology
- Figure 11.2 Clinical Trial Key Insights: Cumulative Distribution by Trial Registration Year, Since 2016
- Figure 11.3 Clinical Trial Key Insights: Distribution by Trial Registration Year and Enrolled Patient Population, Since 2016
- Figure 11.4 Clinical Trial Key Insights: Distribution by Trial Status
- Figure 11.5 Clinical Trial Key Insights: Distribution by Trial Registration Year and Trial Status, Since 2016
- Figure 11.6 Clinical Trial Key Insights: Distribution of Number of Trials by Trial Phase
- Figure 11.7 Clinical Trial Key Insights: Distribution of Enrolled Patient Population by Trial Phase
- Figure 11.8 Clinical Trial Key Insights: Year-wise Distribution by Number of Trials and Trial Phase, Since 2016
- Figure 11.9 Clinical Trial Key Insights: Year-wise Distribution by Number of Enrolled Patient Population and Trial Phase, Since 2016
- Figure 11.10 Clinical Trial Key Insights: Distribution by Type of Sponsor / Collaborator
- Figure 11.11 Clinical Trial Key Insights: Geographical Distribution by Number of Clinical Trials
- Figure 11.12 Clinical Trial Key Insights: Geographical Distribution by Enrolled Patient Population
- Figure 12.1 Analysis of Demand for Clinical Trial Participants: Scope and Methodology
- Figure 12.2 Global Demand for Clinical Trial Participants, Till 2035 (Million Patients)
- Figure 12.3 Global Demand for Clinical Trial Participants, Till 2035: Distribution by Therapeutic Area (Million Patients)
- Figure 12.4 Global Demand for Clinical Trial Participants, Till 2035: Distribution by Trial Phase (Million Patients)
- Figure 12.5 Global Demand for Clinical Trial Participants, 2021: Distribution by Region (Million Patients)
- Figure 13.1 Global Site Management Organizations Market, Till 2035 (USD Billion)
- Figure 13.2 Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area (USD Billion)
- Figure 13.3 Site Management Organizations Market, Till 2035: Distribution by Trial Phase (USD Billion)
- Figure 13.4 Site Management Organizations Market, 2021: Distribution by Clinical Trial Components (USD Billion)
- Figure 13.5 Site Management Organizations Market, Till 2035: Distribution by Type of Intervention (USD Billion)
- Figure 13.6 Site Management Organizations Market, 2021 and 2035: Distribution by Region (USD Billion)
- Figure 13.7 Site Management Organizations Market, 2021 and 2035: Distribution by Country (USD Billion)
- Figure 13.8 Site Management Organizations Market in North America, Till 2035 (USD Billion)
- Figure 13.9 Site Management Organizations Market in the US, Till 2035 (USD Billion)
- Figure 13.10 Site Management Organizations Market in Canada, Till 2035 (USD Billion)
- Figure 13.11 Site Management Organizations Market in Rest of North America, Till 2035 (USD Billion)
- Figure 13.12 Site Management Organizations Market in Europe, Till 2035 (USD Billion)
- Figure 13.13 Site Management Organizations Market in the UK, Till 2035 (USD Billion)
- Figure 13.14 Site Management Organizations Market in France, Till 2035 (USD Billion)
- Figure 13.15 Site Management Organizations Market in Germany, Till 2035 (USD Billion)
- Figure 13.16 Site Management Organizations Market in Spain, Till 2035 (USD Billion)
- Figure 13.17 Site Management Organizations Market in Italy, Till 2035 (USD Billion)
- Figure 13.18 Site Management Organizations Market in Rest of Europe, Till 2035 (USD Billion)
- Figure 13.19 Site Management Organizations Market in Asia-Pacific, Till 2035 (USD Billion)
- Figure 13.20 Site Management Organizations Market in China, Till 2035 (USD Billion)
- Figure 13.21 Site Management Organizations Market in Korea, Till 2035 (USD Billion)
- Figure 13.22 Site Management Organizations Market in India, Till 2035 (USD Billion)
- Figure 13.23 Site Management Organizations Market in Australia, Till 2035 (USD Billion)
- Figure 13.24 Site Management Organizations Market in Japan, Till 2035 (USD Billion)
- Figure 13.25 Site Management Organizations Market in Israel, Till 2035 (USD Billion)
- Figure 13.26 Site Management Organizations Market in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Figure 13.27 Site Management Organizations Market in Latin America, Till 2035 (USD Billion)
- Figure 13.28 Site Management Organizations Market in MENA, Till 2035 (USD Billion)
- Figure 13.29 Site Management Organizations Market in Rest of the World, Till 2035 (USD Billion)
- Figure 14.1 Concluding Remarks: Overall Market Landscape
- Figure 14.2 Concluding Remarks: Partnerships and Collaborations
- Figure 14.3 Concluding Remarks: Funding and Investment
- Figure 14.4 Concluding Remarks: Clinical Trial Key Insights
- Figure 14.5 Concluding Remarks: Analysis of Demand for Clinical Trial Participants
- Figure 14.6 Concluding Remarks: Market Forecast



