 |
市場調查報告書
商品編碼
1771295
生物樣本採購市場:產業趨勢及全球預測 - 依治療領域、腫瘤生物樣本類型、非腫瘤生物樣本類型及主要地區劃分Biospecimen Procurement Market: Industry Trends and Global Forecasts - Distribution by Therapeutic Area, Type of Biospecimen for Oncological Studies, Type of Biospecimen for Non-Oncological Studies and Key Geographical Regions |
||||||
全球生物樣本採購市場:概覽
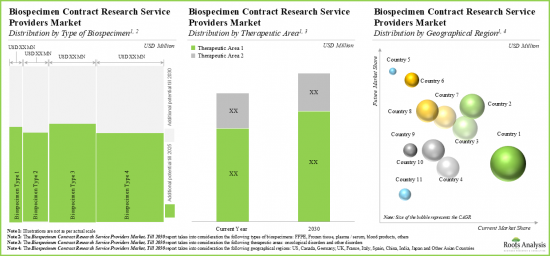
今年全球生物樣本採購市場規模為 5,200萬美元。預計市場在預測期內的年複合成長率為 16%。
市場規模與機會分析依下列參數細分:
治療領域
- 腫瘤疾病
- 其他
腫瘤生物樣本類型
- FFPE
- 冷凍組織
- 血漿/血清
- 其他
生物樣本類型
- 血液製品
- 生物樣本採購
- 其他生物樣本費用
主要地區
- 北美(美國、加拿大)
- 歐洲(德國、法國、義大利、英國、西班牙、其他地區)全球)
- 亞太地區(中國、印度、日本、世界其他地區)
- 世界其他地區
全球生物樣本採購市場:成長與趨勢
生物醫學研究是一個充滿活力且不斷發展的領域,涵蓋目的是闡明疾病和開發有效治療方法的廣泛實驗研究。生物樣本是這個過程的核心,在從早期藥物發現到臨床試驗和大規模流行病學研究的各個階段都發揮關鍵作用。尤其是製藥業日益增多的研發計畫和臨床試驗,推動了對高品質生物樣本的需求。然而,取得和保存高品質的生物樣本並進行準確的分析仍然是製藥業面臨的關鍵挑戰。
確保生物樣本的真實性和高效的可追溯性,需要實施唯一識別碼、先進的資料管理系統和嚴格的品質控制措施。因此,許多研究人員以及製藥和生物製藥公司選擇外包生物樣本的分析服務。事實上,近年來生物樣本研究取得了重大技術進步,改善了生物樣本的儲存和共享,實現了精準的分子研究。此外,該行業採用新的最佳實踐和增強的生物樣本採購、處理和儲存技術,進一步提高了研究品質和真實性的標準。
全球生物樣本採購市場:關鍵洞察
生物樣本採購公司(NCI)是業內領先的生物樣本採購服務提供商,為生物樣本採購(NCI)的研究、開發和營運提供支援。本報告的主要調查結果如下:
- 目前,超過 40 家公司聲稱擁有為各種類型的生物樣本提供廣泛研究和分析服務的必要能力。
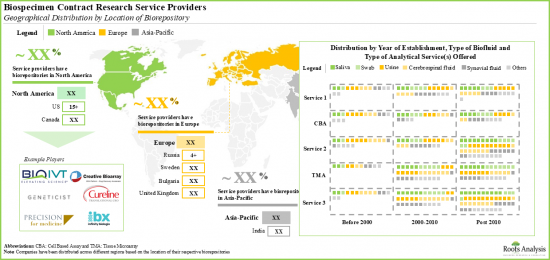
- 生物樣本合約研究服務市場高度分散,參與者種類繁多,包括小型、中型和大型企業。其中,超過 70%的生物樣本採購公司總部位於北美,涵蓋血漿和血清。
- 該領域的大多數小型企業都位於北美,包括 Capital Biosciences、Cureline Translational、iSpecimen 和 Trans-Hit Biomarkers。
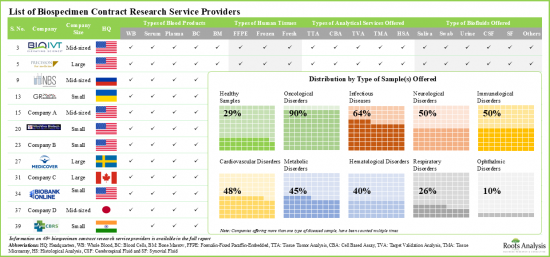
- 約60%的利害關係人聲稱提供尿液和腦脊髓液樣本,有趣的是,超過35%的參與者提供健康樣本。
- 近60%的北美公司同時提供FFPE和新鮮組織。其中超過30%的公司聲稱提供全方位的分析服務。
- 北美正成為這些生物樣本庫的中心。
- 為了獲得競爭優勢,服務提供者擴展其現有能力並增強其提供的服務組合。
- 對生物樣本的需求不斷成長帶來了新的挑戰和機會。為了應對這些挑戰,一些商業銀行已開始提供採購服務。
- 大多數生物樣本庫(約 40%)成立於2009年後。例如 Atreide Biosamples、Ardent Clinical Research Services 和 Dx Biosamples(按字母順序排列)。
- 近 90%的生物樣本庫能夠同時提供體液和人體組織,其中約 70%的生物樣本庫同時提供腫瘤學和傳染病的生物樣本。
- 自2019年以來,該領域的合作活動激增,其中相當一部分包括收購合作夥伴公司的生物樣本相關能力/資產。
- 過去幾年,該領域的合作活動以約 50%的年複合成長率成長,值得注意的是,相當一部分合作是在過去三年中達成的。
- 過去三年達成的合作中有 25%是與總部位於歐洲的參與者達成的。
- 大多數交易(約 30%)是以收購/合併為目的達成的,其次是服務協議,佔比超過20%。
- 北美是生物樣本研究服務提供者的中心,因此來自世界其他地區的創新公司與該地區的公司合作。
- 預計未來十年,大部分服務收入將來自生物樣本研究,主要用於癌症疾病,到2030年,北美仍將是最大的貢獻者。
- 目前,生物樣本合約研究服務的可用性主要限於已開發國家,大部分外包收入均勻分佈在北美(約45%)和亞太地區(約30%)之間。
生物樣本採購市場參與者範例
- BioChain Institute
- BioIVT
- Creative Bioarray
- Discovery Life Sciences
- Infinity BiologiX
- Medicover
- National BioService
- Precision for Medicine
- PrecisionMed
- REPROCELL
本報告調查全球生物樣本採購市場,提供市場概述,以及依治療領域、癌症研究的生物樣本類型、非癌症研究的生物樣本類型的趨勢、依地區劃分的趨勢,和參與市場的公司簡介。
目錄
第1章 前言
第2章 執行摘要
第3章 導論
- 章節概述
- 生物樣本概述
- 生物樣本在藥物研發上的重要性
- 影響生物樣本品質的因素
- 生物樣本管理
- 監理指南
- 生物樣本研究外包
- 未來展望
第4章 市場格局
- 章節概述
- 生物樣本研究服務提供者:市場格局
第5章 競爭格局分析
- 章節概述
- 研究方法
- 關鍵參數
- 競爭分析:擁有生物樣本庫的生物樣本研究服務提供者
- 競爭分析:未擁有生物樣本庫的生物樣本研究服務提供者
第6章 北美生物樣本服務提供者:公司簡介
- 章節概述
- BioChain Institute
- BioIVT
- Creative Bioarray
- Discovery Life Sciences
- Infinity BiologiX
- Precision for Medicine
- PrecisionMed
第7章 歐洲與亞太地區生物樣本服務提供者:公司簡介
- 章節概述
- Medicover
- National BioService
- REPROCELL
第8章 合作夥伴關係與合作
- 章節概述
- 合作模式
- 生物樣本研究服務:合作夥伴關係與合作
- 區域分析
第9章 個案研究:商業生物樣本庫市場格局
- 章節概述
- 商業生物樣本庫:市場格局
- 公司競爭力分析
第10章 關鍵洞察
第11章 市場預測與機會分析
- 第概述
- 預測研究方法與關鍵假設
- 2030年生物樣本研究服務市場整體規模
- 2030年生物樣本研究服務市場:依生物樣本類型
- 2030年生物樣本研究服務市場:依治療領域
- 2030年生物樣本研究服務市場:依主要地區
第12章 結論
第13章 高層洞察
第14章 附錄1:表格資料
第15章 附錄2:公司與組織清單
GLOBAL BIOSPECIMEN PROCUREMENT MARKET: OVERVIEW
As per Roots Analysis, the global biospecimen procurement market valued at USD 52 million in the current year is anticipated to grow at a CAGR of 16% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Therapeutic Area
- Oncological Disorders
- Other Disorders
Type of Biospecimen for Oncological Studies
- FFPE
- Frozen Tissue
- Plasma / Serum
- Other Biospecimens
Type of Biospecimen for Non-Oncological Studies
- Blood Products
- Human Tissues
- Other Biospecimens
Key Geographical Regions
- North America (US and Canada)
- Europe (Germany, France, Italy, UK, Spain and Other European Countries)
- Asia-Pacific (China, India, Japan and Other Asia-Countries)
- Rest of the World
GLOBAL BIOSPECIMEN PROCUREMENT MARKET: GROWTH AND TRENDS
Biomedical research is a dynamic and evolving field that encompasses a broad spectrum of experimental studies aimed at understanding diseases and developing effective treatments. Central to this process are biospecimens, which play a vital role from the initial stages of drug discovery to clinical trials and large-scale epidemiological studies. Notably, the growing number of research and development initiatives in the pharmaceutical industry, and the rising number of clinical trials has led to an increasing demand for high-quality biological samples. However, obtaining and preserving high-quality biospecimens and conducting accurate analyses remains a significant challenge within the pharmaceutical industry.

Ensuring reliable and efficient traceability of biospecimens requires the implementation of unique identifiers, sophisticated data management systems, and rigorous quality control measures. As a result, several researchers and pharmaceutical and biopharmaceutical players have opted to outsource their biospecimen analytical services. In fact, recently there has been significant technological progress in biospecimen research, which enabled precise molecular studies with improved preservation and sharing of biological samples. Further, the industry has also adopted new best practices and enhanced techniques for the procurement, processing, and storage of biospecimens, further elevating the standards of research quality and reliability.
GLOBAL BIOSPECIMEN PROCUREMENT MARKET: KEY INSIGHTS
The report delves into the current state of global biospecimen procurement market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, more than 40 companies claim to have the required capabilities to offer a wide range of research and analytical services for various types of biospecimens.
- The biospecimen contract research services market is highly fragmented, featuring a mix of small, mid-sized and large players. Of these, more than 70% of the biospecimen contract research service providers are headquartered in North America and majority claim of offer services for both plasma and serum.
- Most of the small players in this domain are based in North America; examples include Capital Biosciences, Cureline Translational, iSpecimen and Trans-Hit Biomarkers.
- Around 60% of the stakeholders claim to offer both urinary and cerebrospinal fluid samples; interestingly, over 35% of players offer healthy samples.
- Close to 60% of players based in North America offer both FFPE and fresh tissues; more than 30% of these players claim to provide all types of analytical services.
- Several service providers have established in-house biorepositories in order to cater to the demand for various types of biospecimens across the globe; North America has emerged as the hub for these biorepositories.

- In order to gain a competitive edge, service providers are expanding their existing capabilities to enhance their portfolio of offerings.
- The increasing demand for biospecimens brings new challenges and opportunities along with it; to deal with such challenges, several commercial banks have stepped in to offer procurement services.
- Majority (~40%) of biobanks were established post-2009; examples include (in alphabetical order) Atreide Biosamples, Ardent Clinical Research Services and Dx Biosamples.
- Close to 90% of the biobanks have capabilities to offer both biofluids and human tissues; of this, ~70% offer biospecimens for both oncological disorders and infectious diseases.
- Since 2019, there has been a surge in the partnership activity in this domain; a relatively higher proportion of instances involved the acquisition of biospecimen-related capabilities / assets of the partner firm.
- The partnership activity in this domain has increased at a CAGR of around 50% in the past few years; it is worth mentioning that a significant proportion of the partnerships were inked in the last three years.
- 25% of the partnerships inked in the last three years were signed by players headquartered in Europe.
- Majority of the deals (~30%) were inked for the acquisition / merger purposes, followed by service agreements, accounting for more than 20% of the partnerships.
- With North America being the hub for biospecimen research service providers, several innovators from other regions of the world have partnered with the players based in this region.
- Over the next decade, the majority of service revenues are expected to be generated from biospecimen research primarily focused on oncological disorders; North America is likely to remain the largest shareholder till 2030.

- Presently, the use of biospecimen contract research services is largely restricted to the developed nations, and the majority of revenues generated via outsourcing are well distributed between North America (~45%) and Asia-Pacific (~30%).
Example Players in the Biospecimen Procurement Market
- BioChain Institute
- BioIVT
- Creative Bioarray
- Discovery Life Sciences
- Infinity BiologiX
- Medicover
- National BioService
- Precision for Medicine
- PrecisionMed
- REPROCELL
GLOBAL BIOSPECIMEN PROCUREMENT MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global biospecimen procurement market, focusing on key market segments, including [A] therapeutic area, [B] type of biospecimen for oncological studies, [C] type of biospecimen for non-oncological studies and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of the companies that claim to offer research services related to biospecimens, considering various parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] types of blood products offered, [E] types of human tissues offered, [F] types of biofluids offered, [G] types of other biospecimens offered, [H] types of analytical services offered, [I] types of samples offered, [J] types of accreditations received and [K] geographical reach.
- Company Competitiveness Analysis: A comprehensive company competitive analysis of biospecimen contract research service providers, examining factors, such as [A] portfolio strength, [B] service strength and [C] supplier strength.
- Company Profiles: In-depth profiles of companies offering a wide range of research services for the biospecimens, focusing on [A] company overview, [B] biospecimen related services offered, [C] types of blood products offered, [D] types of human tissues offered, [E] types of other biospecimens offered, [F] types of analytical services offered, [G] types of samples offered, [H] geographical distribution of players offering biospecimen research services and [I] recent developments and an informed future outlook.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the biospecimen procurement market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of collaborator, [D] type of service(s) offered, [E] types of biospecimen(s) handled, [F] therapeutic area, [G] most active players (in terms of the number of partnerships signed) and [H] geography.
- Market Landscape of Commercial Biobanks Case Study: A detailed analysis of the overall landscape of biospecimen procurement service providers (commercial biobanks), based on various parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] types of services offered, [E] types of biospecimens handled, and [F] therapeutic area. Additionally, the section presents a competitive analysis of the commercial biobanks based on the availability of their respective biorepositories.
- Key Insights: A detailed analysis of biospecimen contract research service providers, focusing on four analysis , such as [A] a grid analysis based on the year of establishment and type of analytical services offered, [B] a comprehensive analysis based on the therapeutic area and most active players, [C] an analysis based on the company size and therapeutic area, and [D] a world map representation highlighting the regional distribution of biospecimen research service providers, based on the locations of their biorepositories.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview of Biospecimen
- 3.3. Importance of Biospecimen in Drug Discovery and Research
- 3.4. Factors Affecting the Quality of Biospecimen
- 3.5. Biospecimen Management
- 3.6. Regulatory Guidelines
- 3.7. Outsourcing in Biospecimen Research
- 3.7.1. Role of Contract Research Organizations in Biospecimen Procurement
- 3.7.2. Types of Services Offered by Contract Research Organizations
- 3.8. Future Perspective
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Biospecimen Research Service Providers: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Type of Blood Product(s) Offered
- 4.2.5. Analysis by Type of Human Tissue(s) Offered
- 4.2.6. Analysis by Type of Biofluid(s) and Other Biospecimen(s) Offered
- 4.2.7. Analysis of Type of Analytical Service(s) Offered
- 4.2.8. Analysis by Type of Sample(s) Offered
- 4.2.9. Analysis of Accreditation(s) Received
- 4.2.10. Analysis by Geographical Reach
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Methodology
- 5.3. Key Parameters
- 5.4. Competitiveness Analysis: Biospecimen Research Service Providers with Biorepositories
- 5.4.1. Companies Offering Biospecimen Research Services in North America
- 5.4.2. Companies Offering Biospecimen Research Services in Europe and Asia-Pacific
- 5.5. Competitiveness Analysis: Biospecimen Research Service Providers without Biorepositories
- 5.5.1. Companies Offering Biospecimen Research Services in North America
- 5.5.2. Companies Offering Biospecimen Research Services in Europe
6. BIOSPECIMEN SERVICE PROVIDERS IN NORTH AMERICA: COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. BioChain Institute
- 6.2.1. Company Overview
- 6.2.2. Biospecimen Related Service Portfolio
- 6.2.3. Recent Developments and Future Outlook
- 6.3. BioIVT
- 6.3.1. Company Overview
- 6.3.2. Biospecimen Related Service Portfolio
- 6.3.3. Recent Developments and Future Outlook
- 6.4. Creative Bioarray
- 6.4.1. Company Overview
- 6.4.2. Biospecimen Related Service Portfolio
- 6.4.3. Recent Developments and Future Outlook
- 6.5. Discovery Life Sciences
- 6.5.1. Company Overview
- 6.5.2. Biospecimen Related Service Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. Infinity BiologiX
- 6.6.1. Company Overview
- 6.6.2. Biospecimen Related Service Portfolio
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Precision for Medicine
- 6.7.1. Company Overview
- 6.7.2. Biospecimen Related Service Portfolio
- 6.7.3. Recent Developments and Future Outlook
- 6.8. PrecisionMed
- 6.8.1. Company Overview
- 6.8.2. Biospecimen Related Service Portfolio
- 6.8.3. Recent Developments and Future Outlook
7. BIOSPECIMEN SERVICE PROVIDERS IN EUROPE AND ASIA-PACIFIC: COMPANY PROFILES
- 7.1. Chapter Overview
- 7.2. Medicover
- 7.2.1. Company Overview
- 7.2.2. Biospecimen Related Service Portfolio
- 7.2.3. Recent Developments and Future Outlook
- 7.3. National BioService
- 7.3.1. Company Overview
- 7.3.2. Biospecimen Related Service Portfolio
- 7.3.3. Recent Developments and Future Outlook
- 7.4. REPROCELL
- 7.4.1. Company Overview
- 7.4.2. Biospecimen Related Service Portfolio
- 7.4.3. Recent Developments and Future Outlook
8. PARTNERSHIPS AND COLLABORATIONS
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Biospecimen Research Services: Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type of Collaborator
- 8.3.4. Analysis by Type of Service(s) Offered
- 8.3.5. Analysis by Type of Biospecimen(s) Handled
- 8.3.6. Analysis by Therapeutic Area
- 8.3.7. Most Active Players: Analysis by Number of Partnerships
- 8.4. Geographical Analysis
- 8.4.1. Intercontinental and intracontinental Agreements
9. CASE STUDY: MARKET LANDSCAPE OF COMMERCIAL BIOBANKS
- 9.1. Chapter Overview
- 9.2. Commercial Biobanks: Overall Market Landscape
- 9.2.1. Analysis by Year of Establishment
- 9.2.2. Analysis by Company Size
- 9.2.3. Analysis by Location of Headquarters
- 9.2.4. Analysis by Types of Service(s) Offered
- 9.2.5. Analysis by Types of Biospecimen(s) Handled
- 9.2.6. Analysis by Therapeutic Area
- 9.3. Company Competitiveness Analysis
- 9.3.1. Methodology and Key Parameters
- 9.3.2. Competitiveness Analysis: Commercial Biobanks with Biorepositories
- 9.3.3. Competitiveness Analysis: Commercial Biobanks without Biorepositories
10. KEY INSIGHTS
- 10.1. Chapter Overview
- 10.2. Grid Analysis: Analysis by Year of Establishment and Type of Analytical Service(s) Offered
- 10.3. Analysis by Company Size and Therapeutic Area
- 10.4. Most Active Players: Analysis by Therapeutic Area and
- 10.5. World Map Representation: Analysis by Location of In-House Biorepositories
11. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Overall Biospecimen Research Services Market, Till 2030
- 11.3.1. Biospecimen Research Services Market, Till 2030: Distribution by Type of Biospecimen
- 11.3.2. Biospecimen Research Services Market, Till 2030: Distribution by Therapeutic Area
- 11.3.3. Biospecimen Research Services Market, Till 2030: Distribution by Key Geographical Regions
- 11.3.3.1. Biospecimen Research Services Market in North America, Till 2030: Distribution by Countries
- 11.3.3.2. Biospecimen Research Services Market in North America, Till 2030: Distribution by Type of Biospecimen
- 11.3.3.3. Biospecimen Research Services Market in North America, Till 2030: Distribution by Therapeutic Area
- 11.3.3.4. Biospecimen Research Services Market in Europe, Till 2030: Distribution by Countries
- 11.3.3.5. Biospecimen Research Services Market in Europe, Till 2030: Distribution by Type of Biospecimen
- 11.3.3.6. Biospecimen Research Services Market in Europe, Till 2030: Distribution by Therapeutic Area
- 11.3.3.4. Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Countries
- 11.3.3.5. Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Type of Biospecimen
- 11.3.3.6. Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Therapeutic Area
12. CONCLUSION
- 12.1. Chapter Overview
13. EXECUTIVE INSIGHTS
- 13.1. Chapter Overview
- 13.2. Company A
- 13.2.1. Overview of Company / Organization
- 13.2.2. Interview Transcript: Managing Director and Business Development Head
- 13.3. Company B
- 13.3.1. Overview of Company / Organization
- 13.3.2. Interview Transcript: Director of Business Development
14. APPENDIX 1: TABULATED DATA
15. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 4.1 Biospecimen Research Service Providers: List of Companies
- Table 4.2 Biospecimen Contract Research Service Providers: Information on Type of Blood Product(s) Offered
- Table 4.3 Biospecimen Contract Research Service Providers: Information on Type of Human Tissue(s) Offered
- Table 4.4 Biospecimen Contract Research Service Providers: Information on Type of Biofluid(s) and Other Biospecimen offered
- Table 4.5 Biospecimen Contract Research Service Providers: Information on Type of Analytical Service(s) Offered
- Table 4.6 Biospecimen Contract Research Service Providers: Information on Type of Sample(s) Offered
- Table 4.7 Biospecimen Contract Research Service Providers: Information on Type of Accreditation(s) Received
- Table 4.8 Biospecimen Contract Research Service Providers: Information on Geographical Reach
- Table 6.1 Biospecimen Service Providers in North America: List of Profiled Companies
- Table 6.2 BioChain Institute: Company Snapshot
- Table 6.3 Biospecimen Related Service Portfolio
- Table 6.4 BioChain Institute: Recent Developments and Future Outlook
- Table 6.5 BioIVT: Company Snapshot
- Table 6.6 Biospecimen Related Service Portfolio
- Table 6.7 BioIVT: Recent Developments and Future Outlook
- Table 6.8 Creative Bioarray: Company Snapshot
- Table 6.9 Biospecimen Related Service Portfolio
- Table 6.10 Creative Bioarray: Recent Developments and Future Outlook
- Table 6.11 Discovery Life Sciences: Company Snapshot
- Table 6.12 Biospecimen Related Service Portfolio
- Table 6.13 Discovery Life Sciences: Recent Developments and Future Outlook
- Table 6.14 Infinity BiologiX: Company Snapshot
- Table 6.15 Biospecimen Related Service Portfolio
- Table 6.16 Infinity BiologiX: Recent Developments and Future Outlook
- Table 6.17 Precision for Medicine: Company Snapshot
- Table 6.18 Biospecimen Related Service Portfolio
- Table 6.19 Precision for Medicine: Recent Developments and Future Outlook
- Table 6.20 PrecisionMed: Company Snapshot
- Table 6.21 Biospecimen Related Service Portfolio
- Table 6.22 PrecisionMed: Recent Developments and Future Outlook
- Table 7.1 Medicover: Company Snapshot
- Table 7.2 Biospecimen Related Service Portfolio
- Table 7.3 Medicover: Recent Developments and Future Outlook
- Table 7.4 National BioService: Company Snapshot
- Table 7.5 Biospecimen Related Service Portfolio
- Table 7.6 National BioService: Recent Developments and Future Outlook
- Table 7.7 REPROCELL: Company Snapshot
- Table 7.8 Biospecimen Related Service Portfolio
- Table 7.9 REPROCELL: Recent Developments and Future Outlook
- Table 9.1 Biospecimen Contract Research Service Providers: Partnerships and Collaborations, Since 2015
- Table 9.2 Partnerships and Collaborations: Information on Type of Service(s) Offered, Type of Biospecimens(s) Handled and Therapeutic Area, Since 2015
- Table 10.1 Commercial Biobanks: List of Companies
- Table 10.2 Commercial Biobanks: Information on Type of Service(s) Offered
- Table 10.2 Commercial Biobanks: Information on Type of Biospecimen(s) Handled
- Table 10.3 Commercial Biobanks: Information on Therapeutic Areas
- Table 14.1 Biospecimen Contract Research Service Providers: Distribution by Year of Establishment
- Table 14.2 Biospecimen Contract Research Service Providers: Distribution by Company Size
- Table 14.3 Biospecimen Contract Research Service Providers: Distribution by Location of Headquarters
- Table 14.4 Biospecimen Contract Research Service Providers: Distribution by Type of Blood Product(s) Offered and Type of Sample(s) Offered
- Table 14.5 Biospecimen Contract Research Service Providers: Distribution by Type of Human Tissue(s) Offered
- Table 14.6 Biospecimen Contract Research Service Providers: Distribution by Type of Biofluid(s) Offered
- Table 14.7 Biospecimen Contract Research Service Providers: Distribution by Type of Analytical Service(s) Offered
- Table 14.8 Biospecimen Contract Research Service Providers: Distribution by Type of Sample(s) Offered
- Table 14.9 Biospecimen Contract Research Service Providers: Distribution by Accreditation(s) Received
- Table 14.10 Biospecimen Contract Research Service Providers: Distribution by Type of Analytical Service(s) Offered and Geographical Reach
- Table 14.11 Partnerships and Collaborations: Distribution by Year of Partnership
- Table 14.12 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 14.13 Partnerships and Collaborations: Distribution by Type of Collaborator
- Table 14.14 Partnerships and Collaborations: Distribution by Type of Service(s) Offered
- Table 14.15 Partnerships and Collaborations: Distribution by Type of Biospecimen(s) Handled
- Table 14.16 Partnerships and Collaborations: Distribution by Therapeutic Area
- Table 14.17 Partnerships and Collaborations: Distribution of Most Active Players by Number of Partnerships
- Table 14.18 Partnerships and Collaborations: Geographical Analysis
- Table 14.19 Partnerships and Collaborations: Distribution Intercontinental and Intracontinental Agreements
- Table 14.20 Commercial Biobanks: Distribution by Year of Establishment
- Table 14.21 Commercial Biobanks: Distribution by Company Size
- Table 14.22 Commercial Biobanks: Distribution by Location of Headquarters
- Table 14.23 Commercial Biobanks: Distribution by Types of Service(s) Offered
- Table 14.24 Commercial Biobanks: Distribution by Types of Biospecimen(s) Handled
- Table 14.25 Commercial Biobanks: Distribution by Therapeutic Area
- Table 14.26 Biospecimen Service Providers: Distribution by Type of Company Size and Therapeutic Area
- Table 14.27 Overall Biospecimen Research Services Market, Till 2030 (USD Million)
- Table 14.28 Biospecimen Research Services Market, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Table 14.29 Biospecimen Research Services Market, Till 2030: Distribution by Therapeutic Area (USD Million)
- Table 14.30 Biospecimen Research Services Market, Till 2030: Distribution by Key Geographical Regions (USD Million)
- Table 14.31 Biospecimen Research Services Market in North America, Till 2030: Distribution by Countries (USD Million)
- Table 14.32 Biospecimen Research Services Market in North America, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Table 14.33 Biospecimen Research Services Market in North America, Till 2030: Distribution by Therapeutic Area (USD Million)
- Table 14.34 Biospecimen Research Services Market in Europe, Till 2030: Distribution by Countries (USD Million)
- Table 14.35 Biospecimen Research Services Market in Europe, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Table 14.36 Biospecimen Research Services Market in Europe, Till 2030: Distribution by Therapeutic Area (USD Million)
- Table 14.37 Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Countries (USD Million)
- Table 14.38 Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Table 14.39 Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Therapeutic Area (USD Million)
List of Figures
- Figure 2.1 Executive Summary: Current Market Landscape
- Figure 2.2 Executive Summary: Partnerships and Collaborations
- Figure 2.2 Executive Summary: Current Landscape of Commercial Biobanks
- Figure 2.3 Executive Summary: Market Forecast and Opportunity Analysis
- Figure 3.1 Factors Affecting the Quality of Biospecimen
- Figure 3.2 Lifecycle of a Biospecimen
- Figure 3.3 Clinical Trial related Services offered by CROs
- Figure 4.1 Biospecimen Contract Research Service Providers: Distribution by Year of Establishment
- Figure 4.2 Biospecimen Contract Research Service Providers: Distribution by Company Size
- Figure 4.3 Biospecimen Contract Research Service Providers: Distribution by Location of Headquarters
- Figure 4.4 Biospecimen Contract Research Service Providers: Distribution by Type of Blood Product(s) Offered and Type of Sample(s) Offered
- Figure 4.5 Biospecimen Contract Research Service Providers: Distribution by Type of Human Tissue(s) Offered
- Figure 4.6 Biospecimen Contract Research Service Providers: Distribution by Type of Biofluid(s) Offered
- Figure 4.7 Biospecimen Contract Research Service Providers: Distribution by Type of Analytical Service(s) Offered
- Figure 4.8 Biospecimen Contract Research Service Providers: Distribution by Type of Sample(s) Offered
- Figure 4.9 Biospecimen Contract Research Service Providers: Distribution by Accreditation(s) Received
- Figure 4.10 Biospecimen Contract Research Service Providers: Distribution by Type of Analytical Service(s) Offered and Geographical Reach
- Figure 5.1 Company Competitiveness Analysis: Biospecimen Research Service Providers based in North America (With Biorepositories)
- Figure 5.2 Company Competitiveness Analysis: Biospecimen Research Service Providers based in Europe and Asia-Pacific (With Biorepositories)
- Figure 5.3 Company Competitiveness Analysis: Biospecimen Research Service Providers based in North America (Without Biorepositories)
- Figure 5.4 Company Competitiveness Analysis: Biospecimen Research Service Providers based in Europe and Asia-Pacific (Without Biorepositories)
- Figure 8.1 Partnerships and Collaborations: Distribution by Year of Partnership
- Figure 8.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 8.3 Partnerships and Collaborations: Distribution by Type of Collaborator
- Figure 8.4 Partnerships and Collaborations: Distribution by Type of Service(s) Offered
- Figure 8.5 Partnerships and Collaborations: Distribution by Type of Biospecimen(s) Handled
- Figure 8.6 Partnerships and Collaborations: Distribution by Therapeutic Area
- Figure 8.7 Partnerships and Collaborations: Distribution of Most Active Players by Number of Partnerships
- Figure 8.8 Partnerships and Collaborations: Geographical Analysis
- Figure 8.9 Partnerships and Collaborations: Distribution of Intercontinental and Intracontinental Agreements
- Figure 9.1 Commercial Biobanks: Distribution by Year of Establishment
- Figure 9.2 Commercial Biobanks: Distribution by Company Size
- Figure 9.3 Commercial Biobanks: Distribution by Location of Headquarters
- Figure 9.4 Commercial Biobanks: Distribution by Type of Service(s) Offered
- Figure 9.5 Commercial Biobanks: Distribution by Type of Biospecimen(s) Handled
- Figure 9.6 Commercial Biobanks: Distribution by Therapeutic Area
- Figure 9.7 Competitiveness Analysis: Commercial Biobanks with Biorepositories
- Figure 9.8 Competitiveness Analysis: Commercial Biobanks without Biorepositories
- Figure 10.1 Grid Analysis: Distribution by Year of Establishment and Type of Analytical Services Offered
- Figure 10.2 Biospecimen Service Providers: Distribution by Type of Company Size and Therapeutic Area
- Figure 10.3 Most Active Players: Distribution by Therapeutic Area
- Figure 10.4 World Map Representation: Distribution by Location of In-House Biorepositories
- Figure 11.1 Overall Biospecimen Research Services Market, Till 2030 (USD Million)
- Figure 11.2 Biospecimen Research Services Market, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Figure 11.3 Biospecimen Research Services Market, Till 2030: Distribution by Therapeutic Area (USD Million)
- Figure 11.4 Biospecimen Research Services Market, Till 2030: Distribution by Key Geographical Regions (USD Million)
- Figure 11.5 Biospecimen Research Services Market in North America, Till 2030: Distribution by Countries (USD Million)
- Figure 11.6 Biospecimen Research Services Market in North America, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Figure 11.7 Biospecimen Research Services Market in North America, Till 2030: Distribution by Therapeutic Area (USD Million)
- Figure 11.8 Biospecimen Research Services Market in Europe, Till 2030: Distribution by Countries (USD Million)
- Figure 11.9 Biospecimen Research Services Market in Europe, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Figure 11.10 Biospecimen Research Services Market in Europe, Till 2030: Distribution by Therapeutic Area (USD Million)
- Figure 11.11 Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Countries (USD Million)
- Figure 11.12 Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Type of Biospecimen (USD Million)
- Figure 11.13 Biospecimen Research Services Market in Asia-Pacific, Till 2030: Distribution by Therapeutic Area (USD Million)
- Figure 12.1 Concluding Remarks: Current Biospecimen Contract Research Service Providers Market Landscape Summary
- Figure 12.2 Concluding Remarks: Partnerships and Collaborations
- Figure 12.3 Concluding Remarks: Market Forecast and Opportunity Analysis







