 |
市場調查報告書
商品編碼
1771290
DNA損害反應市場:產業趨勢和全球預測 - 各適應疾病,各治療領域,目標分子,各分子類型,各給藥途徑,主要各地區DNA Damage Response Market: Industry Trends and Global Forecasts - Distribution by Target Disease Indication, Therapeutic Area, Target Molecule, Type of Molecule, Route of Administration, and Key Geographical Regions |
||||||
全球DNA損傷反應市場:概覽
今年全球DNA損傷反應市場規模達1,300萬美元。預計在預測期內,該市場將保持良好的複合年增長率。
市場區隔與機會分析依下列參數細分:
適應疾病
- 急性骨髓性白血病
- 新冠肺炎 (COVID-19)
- 糖尿病黃斑水腫
- 間皮瘤
- 骨髓增生異常症候群
- 非鱗狀非小細胞肺癌
- 攝護腺癌
- 子宮漿液性癌
治療領域
- 骨髓惡性腫瘤
- 固體癌
- 其他
目標分子
- APE1/Ref-1
- 酪蛋白激梅2
- CHK-1
- C-Tak
- DHODH
- MAPKAPK2
- p53
- WEE 1
分子類型
- 生技藥品
- 低分子
給藥途徑
- 口服藥
- 靜脈注射藥
主要地區
- 北美(美國,加拿大)
- 歐洲(丹麥,法國,德國,義大利,西班牙,英國)
- 亞太地區(澳洲,新加坡,韓國)
全球DNA損傷反應市場:成長與趨勢
DNA損傷反應 (DDR) 由一個協調的路徑網絡組成,這些路徑不僅促進DNA損傷修復,還能活化細胞週期檢查點。這會導致細胞週期在關鍵階段停滯,以維持整個基因組的完整性。值得注意的是,如果損傷無法修復,這個複雜的系統確保細胞要麼在分裂前修復遺傳物質,要麼促進程序性細胞死亡,以防止突變的傳播。此外,DDR 對常規療法的高特異性和敏感性以及低脫靶毒性使 DDR 成為包括腫瘤和非腫瘤疾病在內的多種臨床疾病的有希望的治療標靶。因此,世界各地的研究人員正在開發 DDR 抑制劑,以對抗 DDR 介導的對 DNA 損傷抗癌療法的抗藥性,並透過靶向替代途徑來利用癌症中的 DDR 功能障礙。
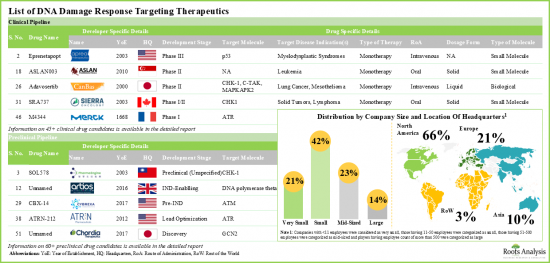
值得注意的是,目前已有四種針對 DNA 損傷修復過程的聚 ADP-核糖聚合酶 (PARP) 抑制劑獲準用於治療晚期癌症。此外,全球藥物開發商正在研究 DNA 損傷反應路徑中的其他分子標靶,包括 ATM、ATR、CHK1 和 WEE1。
全球 DNA 損傷反應市場:關鍵洞察
本報告深入探討了全球 DNA 損傷反應市場的現狀,並揭示了該行業的潛在成長機會。報告的主要發現包括:
- 目前,近45家公司正在開發針對DNA損傷反應(DDR)的療法,以治療各種臨床適應症。
- 大多數候選藥物處於早期開發階段,主要針對各種癌症疾病特有的生物分子表位。
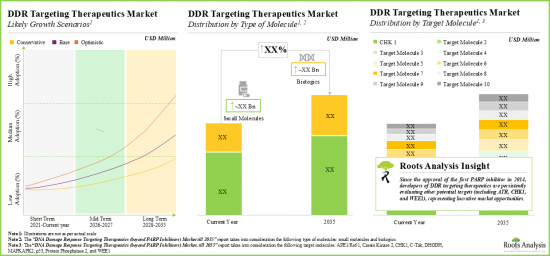
- 針對DDR的療法的臨床前研發管線強勁且不斷成長。這些候選藥物中的大多數(超過75%)是小分子。
- 超過70%的針對ATR的候選藥物正在臨床試驗中,其中約55%的此類幹預措施設計為口服給藥。
- 約65%的用於治療實體瘤的DDR標靶藥物已在臨床前試驗中得到概念驗證。
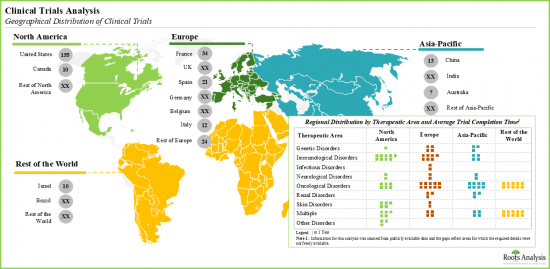
- 自2010年以來,已有超過220項臨床試驗註冊,用於評估DDR標靶療法的療效,儘管亞太地區此類試驗的平均完成時間相對較短。
- 從頂尖期刊發表的大量科學論文可以看出,該領域的創新蓬勃發展。目前的重點似乎是尋找新的靶點,尤其是針對不同類型的癌症。
- 過去一年,該領域的論文數量顯著增加,約30%的論文發表於2021年後。
- 根據已發表的論文/文章,目前的研究重點是ATR、ADP、ATR和HSP等標靶分子。
- 針對癌症(包括白血病、肺癌和卵巢癌)的DDR標靶療法的研究明顯增加。
- 從早期研發到藥物上市,有多個參數會影響定價和採用率。研發人員必須綜合考慮所有這些因素,才能在競爭中保持領先地位。
- 為了獲得競爭優勢並贏得龐大的消費者群體,創新者必須了解直接和間接影響其產品採用率和定價的因素。
- 鑑於前景光明的療法研發管線和令人鼓舞的臨床研究結果,預計到2035年,DDR標靶療法市場將實現顯著的年化成長。
- 預期的市場機會可能在各種目標疾病適應症、給藥途徑和主要地區呈現高度多樣化。
DNA損害反應市場參與企業案例
- Aprea Therapeutics
- AstraZeneca
- Chordia Therapeutics
- Mission Therapeutics
- Repare Therapeutics
- Senhwa Biosciences
全球DNA損害反應市場
- 市場規模和機會分析:本研究報告對全球DNA損傷反應市場進行了深入分析,重點關注關鍵細分市場,例如[A]適應症、[B]治療領域、[C]目標分子、[D]分子類型、 [E] 給藥途徑和 [F] 重點區域。
- 市場格局:對DNA損傷反應標靶療法進行全面評估,考慮各種參數,例如A]開發階段、[B]適應症、[C]治療領域、[D]標靶分子、[E]分子類型、[F]治療類型、[G]劑型、[H]給藥途徑、[I]特定藥物名稱(如有)。此外,也根據[A]成立年份、[B]公司規模(員工人數)和[C]總部所在地,對從事DNA損傷反應標靶療法開發的公司進行全面評估。
- 關鍵洞察:使用四個圖表對當代市場趨勢進行深入分析,包括A]比較DNA損傷反應市場主要公司的氣泡分析、[B]基於目標治療領域和公司規模的DNA損傷反應靶向療法開發公司的分析、[C]重點介紹DNA損傷反應市場開發公司區域分佈的詳細分析,以及[D]顯示DNA損傷反應靶向療法分佈的綜合分析。
- 公司簡介:A] 詳細介紹從事 DNA 損傷反應標靶療法開發的公司,重點關注 [A] 公司概況、[B] 每種候選藥物的詳細資訊以及 [C] 近期趨勢和未來展望。
- 臨床試驗分析:基於各種相關參數,對 250 多個已完成、正在進行和計劃中的各種 DNA 損傷反應標靶療法臨床試驗進行詳細分析,這些參數包括 A] 臨床試驗註冊年份、[B] 入組患者人數、[C] 入組患者性別、[D] 臨床試驗階段、[E] 招募狀態和研究設計、[F] 主要數量(G])組織類型、[H] 熱門治療領域、[i] 臨床試驗的區域分佈。
- 文章分析:基於 A] 出版年份、[B] 出版類型、[C] 主要研究中心、[D] 最受歡迎的作者、[E] 資助金額、[F] 靶點分子、[G] 最受歡迎的期刊,對 150 多篇與 DNA 損傷反應靶向研究相關的同行評審科學文章進行了深入分析。
本報告概述了全球 DNA 損傷反應市場,並提供了詳細的市場分析和市場概況,包括適應症、治療領域、靶點分子、分子類型、按給藥途徑劃分的趨勢、按地區劃分的趨勢以及參與市場的公司概況。
目錄
第1章 序文
第2章 摘要整理
第3章 簡介
- 章概要
- DNA損傷概要
- DNA損傷物質
- DNA損害反應系統
- DNA修復途徑的種類
- 結論
第4章 市場形勢
- 章概要
- DNA損害反應標靶治療藥:臨床實驗平台
- DNA損害反應標靶治療藥:前臨床開發平台
- DNA損害反應標靶治療藥:開發商清單
第5章 重要的洞察
- 章概要
- 組合的強度,開發階段,不同企業規模分析(4D泡泡圖)
- 與治療領域企業規模分別分析(樹地圖表示)
- 總公司所在地分析(世界地圖表示)
- 開發階段,治療領域,分子類型,治療方法的種類,各給藥途徑分析(電網表示)
第6章 企業簡介
- 章概要
- Aprea Therapeutics
- AstraZeneca
- Chordia Therapeutics
- Mission Therapeutics
- Repare Therapeutics
- Senhwa Biosciences
第7章 臨床試驗的分析
- 章概要
- 範圍與調查手法
- DNA損害反應標靶治療藥:臨床試驗分析
第8章 出版物的分析
- 章概要
- 範圍與調查手法
- DNA損害反應標靶治療藥:最近的出版物一覽
第9章 影響藥品定價與應用的關鍵參數分析
- 章概要
- 主要的推動市場要素
- 始祖分析組成架構
第10章 市場預測
- 章概要
- 範圍與限制
- 預測調查手法主要的前提條件
- 到2035年前的全球DNA損害反應標靶治療藥市場
- DNA損害反應標靶治療藥市場:各適應疾病
- DNA損害反應標靶治療藥市場:各治療領域
- DNA損害反應標靶治療藥市場:目標分子
- DNA損害反應標靶治療藥市場:各分子類型
- DNA損害反應標靶治療藥市場:各給藥途徑
- DNA損害反應標靶治療藥市場:各地區
- 各類醫藥品預測銷售額
- 結論
第11章 結論
第12章 附錄I:表格形式資料
第13章 附錄II:企業及組織一覽
GLOBAL DNA DAMAGE RESPONSE MARKET: OVERVIEW
As per Roots Analysis, the global DNA damage response market valued at USD 13 million in the current year is anticipated to grow at a lucrative CAGR during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Disease Indication
- Acute Myeloid Leukemias
- COVID-19
- Diabetic Macular Edemas
- Mesotheliomas
- Myelodysplastic Syndromes
- Non-Squamous Non-Small Cell Lung Cancers
- Prostate Cancers
- Uterine Serous Carcinomas
Therapeutic Area
- Hematological Malignancies
- Solid Tumors
- Other Disorders
Target Molecule
- APE1/Ref-1
- Casein Kinase 2
- CHK-1
- C-Tak
- DHODH
- MAPKAPK2
- p53
- WEE 1
Type of Molecule
- Biologics
- Small Molecule
Route of Administration
- Oral Drugs
- Intravenous Drugs
Key Geographical Regions
- North America (US and Canada)
- Europe (Denmark, France, Germany, Italy, Spain and UK)
- Asia-Pacific (Australia, Singapore and South Korea)
GLOBAL DNA DAMAGE RESPONSE MARKET: GROWTH AND TRENDS
The DNA damage response (DDR) consists of a coordinated network of pathways that not only facilitate the repair of DNA lesions but also activate cell cycle checkpoints. This leads to cell cycle arrest at critical stages in order to maintain the overall genomic integrity. Notably, if the damage is irreparable, this intricate system ensures that cells either repair their genetic material before undergoing division or facilitate programmed cell death to prevent the propagation of mutations. Further, its high specificity and sensitivity to conventional therapies, and low off-target toxicity have made DDR a promising therapeutic target for a broad range of clinical conditions, including both oncological and non-oncological diseases. Consequently, researchers worldwide are developing DDR inhibitors to counter DDR-mediated resistance to DNA-damaging anticancer therapies and to exploit DDR dysfunction in cancer by targeting alternative pathways.

It is worth mentioning that four poly-ADP ribose polymerase (PARP) inhibitor drugs, which target the DNA damage repair process, are currently approved for advanced-stage cancer treatment. Additionally, drug developers worldwide are investigating other molecular targets within the DNA damage response pathway, including ATM, ATR, CHK1, and WEE1.
GLOBAL DNA DAMAGE RESPONSE MARKET: KEY INSIGHTS
The report delves into the current state of global DNA damage response market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, close to 45 companies are engaged in the development of DNA damage response (DDR) targeting therapeutics in order to treat a range of clinical conditions.
- Majority of pipeline drug candidates are in the early phases of development; these are predominantly designed to target epitopes on biological molecules that are a characteristic of various oncological disorders.
- The preclinical pipeline of DDR targeting therapeutics is substantial and growing; majority of these drug candidates (over 75%) are small molecules.
- Over 70% of drug candidates targeting ATR are in clinical trials and around 55% of such interventions are designed for oral administration.
- About 65% of DDR targeting drugs intended to treat solid tumors have already demonstrated preclinical proof of concept.
- Since 2010, over 220 clinical trials have been registered to evaluate the efficacy of DDR targeting therapies; average completion time for such studies was relatively less in Asia-Pacific.

- Innovation in this field is evident across the plethora of scientific articles published in prestigious journals; the current focus appears to be on identification of novel targets, specifically against different types of cancers.
- The number of publications in this domain has increased significantly in the past one year; around 30% of the articles have been published since 2021.
- Published articles / papers indicate that the focus of current research activity is on target molecules, such as ATR, ADP, ATR and HSP.
- There has been an evident increase in research focused on DDR targeting therapies against cancers, such as leukemia, lung and ovarian cancers.
- Several parameters, ranging from initial stages of development to launch of the drug, influence the pricing and adoption rates; developers must consider the combination of all these factors to survive the competition.
- In pursuit of a competitive edge and the successful establishment of a large consumer base, it is imperative for innovators to understand both direct and indirect influences on the adoption and pricing of their respective products.
- Considering the promising development pipeline of therapies and encouraging clinical research outcomes, the DDR targeting therapeutics market is anticipated to grow at a significant annualized till 2035.
- The projected market opportunity is likely to be well-distributed across different target disease indications, routes of administration and key geographical regions.

Example Players in the DNA Damage Response Market
- Aprea Therapeutics
- AstraZeneca
- Chordia Therapeutics
- Mission Therapeutics
- Repare Therapeutics
- Senhwa Biosciences
GLOBAL DNA DAMAGE RESPONSE MARKET
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global DNA damage response market, focusing on key market segments, including [A] target disease indication, [B] therapeutic area, [C] target molecule, [D] type of molecule, [E] route of administration and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of DNA damage response targeting therapeutics, considering various parameters, such as [A] phase of development, [B] target disease indication(s), [C] therapeutic area, [D] target molecule, [E] type of molecule, [F] type of therapy, [G] dosage form, [H] route of administration and [I] special drug designation awarded (if any). Additionally, a comprehensive evaluation of the companies engaged in development of DNA damage response targeting therapeutics, based on [A] year of establishment, [B] company size (in terms of employee count) and [C] location of respective headquarters.
- Key Insights: An insightful analysis of contemporary market trends that have been depicted using four schematic representations, including [A] a bubble analysis comparing the leading players engaged in DNA damage response market, [B] an analysis of DNA damage response targeting therapeutics developers, based on their target therapeutic area and company size, [C] a detailed analysis highlighting the regional distribution of developers engaged in DNA damage response market, and [D] a comprehensive analysis illustrating the distribution of DNA damage response targeting therapeutics.
- Company Profiles: In-depth profiles of companies engaged in the development of DNA damage response targeting therapeutics, focusing on [A] company overview, [B] details related to its respective drug candidates and [C] recent developments and an informed future outlook.
- Clinical Trial Analysis: An in-depth analysis of more than 250 completed, ongoing and planned clinical studies of various DNA damage response targeting therapeutics, based on various relevant parameters, such as [A] trial registration year, [B] number of patients enrolled, [C] gender of patients enrolled, [D] trial phase, [E] recruitment status and study design, leading [F] sponsors / collaborators and leading players (in terms of number of trials conducted), [G] type of organization, [H] popular therapeutic areas and [I] regional distribution of trials.
- Publication Analysis: An insightful analysis of more than 150 peer-reviewed scientific articles related to DNA damage response targeting therapeutics, based on [A] year of publication, [B] type of publication, [C] key research hubs, [D] most popular authors, [E] provision of grant awarded, [F] target molecule, and [G] most popular journals.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview of DNA Damage
- 3.3. DNA Damaging Agents
- 3.4. DNA Damage Response Systems
- 3.4.1. Key Components of DNA Repair Pathways
- 3.5. Types of DNA Repair Pathways
- 3.5.1. Direct Pathways
- 3.5.2. Excision Repair Pathway
- 3.5.2.1. Base Excision Repair Pathway
- 3.5.2.2. Nucleotide Excision Repair Pathway
- 3.5.2.3. Mismatch Repair Pathway
- 3.5.3. Indirect Pathways
- 3.5.3.1. Homologous Recombination (HR) Repair Pathway
- 3.5.3.2. Non-homologous End Joining (NHEJ) Repair Pathway
- 3.6. Concluding Remarks
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. DNA Damage Response Targeting Therapeutics: Clinical Pipeline
- 4.2.1. Analysis by Phase of Development
- 4.2.2. Analysis by Target Disease Indication(s)
- 4.2.3. Analysis by Therapeutic Area
- 4.2.4. Analysis by Target Molecule
- 4.2.5. Analysis by Type of Molecule
- 4.2.6. Analysis by Type of Therapy
- 4.2.7. Analysis by Dosage Form
- 4.2.8. Analysis by Route of Administration
- 4.2.9. Analysis by Special Drug Designation Awarded
- 4.3. DNA Damage Response Targeting Therapeutics: Preclinical Pipeline
- 4.3.1. Analysis by Phase of Development
- 4.3.2. Analysis by Target Disease Indication(s)
- 4.3.3. Analysis by Therapeutic Area
- 4.3.4. Analysis by Type of Molecule
- 4.3.5. Analysis by Type of Therapy
- 4.4 DNA Damage Response Targeting Therapeutics: List of Developers
- 4.4.1. Analysis by Year of Establishment
- 4.4.2. Analysis by Company Size
- 4.4.3. Analysis by Location of Headquarters
- 4.4.4. Leading Developers: Analysis by Number of Proprietary Product Candidates
5. KEY INSIGHTS
- 5.1. Chapter Overview
- 5.2. Analysis by Portfolio Strength, Phase of Development and Company Size (4D Bubble Chart)
- 5.3. Analysis by Therapeutic Area and Company Size (Treemap Representation)
- 5.4. Analysis by Location of Headquarters (World Map Representation)
- 5.5. Analysis by Phase of Development, Therapeutic Area, Type of Molecule, Type of Therapy and Route of Administration (Grid Representation)
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Aprea Therapeutics
- 6.2.1. Company Overview
- 6.2.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.2.3. Recent Developments and Future Outlook
- 6.3. AstraZeneca
- 6.3.1. Company Overview
- 6.3.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.3.3. Recent Developments and Future Outlook
- 6.4. Chordia Therapeutics
- 6.4.1. Company Overview
- 6.4.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.4.3. Recent Developments and Future Outlook
- 6.5. Mission Therapeutics
- 6.5.1. Company Overview
- 6.5.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. Repare Therapeutics
- 6.6.1. Company Overview
- 6.6.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Senhwa Biosciences
- 6.7.1. Company Overview
- 6.7.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.7.3. Recent Developments and Future Outlook
7. CLINICAL TRIALS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. DNA Damage Response Targeting Therapeutics: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Number of Patients Enrolled
- 7.3.3. Analysis by Gender of Patients Enrolled
- 7.3.4. Analysis by Trial Phase
- 7.3.5. Analysis by Recruitment Status
- 7.3.6. Analysis by Study Design
- 7.3.7. Analysis by Type of Sponsor / Collaborator
- 7.3.8. Analysis by Therapeutic Area
- 7.3.9. Reginal Analysis
- 7.3.10. Case Study
- 7.3.11. Most Active Industry Players: Analysis by Number of Clinical Trails
- 7.3.12. Concluding Remarks
8. PUBLICATION ANALYSIS
- 8.1. Chapter Overview
- 8.2. Scope and Methodology
- 8.3. DNA Damage Response Targeting Therapeutics: List of Recent Publications
- 8.3.1. Analysis by Year of Publication
- 8.3.2. Analysis by Type of Publication
- 8.3.3. Emerging Focus Areas
- 8.3.4. Analysis by Key Research Journals
- 8.3.4.1. Most Prominent Journals: Analysis by Number of Publications
- 8.3.4.2. Analysis by Journal Impact Factor
- 8.3.4.3. Most Prominent Journals: Analysis by Journal Impact Factor
- 8.3.5. Analysis by Key Research Hubs
- 8.3.6. Analysis by Target Molecule
- 8.3.6.1. Most Popular Target Molecule: Analysis by Number of Publications
- 8.3.6.2. Analysis by Year and Target Molecule
- 8.3.7. Analysis by Grants Awarded
- 8.3.7.1. Locations of Grant Awarding Organizations: Analysis by Number of Publications
- 8.3.8. Publication Benchmarking Analysis
9. ANALYSIS OF KEY PARAMETERS IMPACTING DRUG PRICING AND ADOPTION
- 9.1. Chapter Overview
- 9.2. Key Market Drivers
- 9.3. Roots Analysis Framework
- 9.3.1. Benchmarking Parameters
- 9.3.2. Methodology
- 9.3.3. Impact on Price and Adoption
- 9.3.4. Impact on Pricing and Adoption of Individual Drugs / Drug Candidates
- 9.3.4.1. Adavosertib
- 9.3.4.2. APX3330
- 9.3.4.3. ASLAN003
- 9.3.4.4. CBP-501
- 9.3.4.5. Eprenetapopt
- 9.3.4.6. Irofulven
- 9.3.4.7. LB-100
- 9.3.4.8. Silmitasertib
- 9.3.4.9. TRC-102
- 9.3.5. Concluding Remarks
10. MARKET FORECAST
- 10.1. Chapter Overview
- 10.2. Scope and Limitations
- 10.3. Forecast Methodology and Key Assumptions
- 10.4. Global DNA Damage Response Targeting Therapeutics Market, Till 2035
- 10.4.1. DNA Damage Response Targeting Therapeutics Market: Distribution by Target Disease Indication
- 10.4.1.1. DNA Damage Response Targeting Therapeutics Market for Acute Myeloid Leukemias, Till 2035
- 10.4.1.2. DNA Damage Response Targeting Therapeutics Market for COVID-19, Till 2035
- 10.4.1.3. DNA Damage Response Targeting Therapeutics Market for Diabetic Macular Edemas, Till 2035
- 10.4.1.4. DNA Damage Response Targeting Therapeutics Market for Mesotheliomas, Till 2035
- 10.4.1.5. DNA Damage Response Targeting Therapeutics Market for Myelodysplastic Syndromes, Till 2035
- 10.4.1.6. DNA Damage Response Targeting Therapeutics Market for Non-Squamous Non-Small Cell Lung Cancers, Till 2035
- 10.4.1.7. DNA Damage Response Targeting Therapeutics Market for Prostate Cancers, Till 2035
- 10.4.1.8. DNA Damage Response Targeting Therapeutics Market for Uterine Serous Carcinomas, Till 2035
- 10.4.2. DNA Damage Response Targeting Therapeutics Market: Distribution by Therapeutic Area
- 10.4.2.1. DNA Damage Response Targeting Therapeutics Market for Hematological Malignancies, Till 2035
- 10.4.2.2. DNA Damage Response Targeting Therapeutics Market for Solid Tumors, Till 2035
- 10.4.2.3. DNA Damage Response Targeting Therapeutics Market for Other Disorders, Till 2035
- 10.4.3. DNA Damage Response Targeting Therapeutics Market: Distribution by Target Molecule
- 10.4.3.1. DNA Damage Response Targeting Therapeutics Market for APE1/Ref-1, Till 2035
- 10.4.3.2. DNA Damage Response Targeting Therapeutics Market for Casein Kinase 2, Till 2035
- 10.4.3.3. DNA Damage Response Targeting Therapeutics Market for CHK-1, Till 2035
- 10.4.3.4. DNA Damage Response Targeting Therapeutics Market for C-Tak, Till 2035
- 10.4.3.5. DNA Damage Response Targeting Therapeutics Market for DHODH, Till 2035
- 10.4.3.6. DNA Damage Response Targeting Therapeutics Market for MAPKAPK2, Till 2035
- 10.4.3.7. DNA Damage Response Targeting Therapeutics Market for p53, Till 2035
- 10.4.3.8. DNA Damage Response Targeting Therapeutics Market for Protein Phosphatase 2A, Till 2035
- 10.4.3.9. DNA Damage Response Targeting Therapeutics Market for WEE1, Till 2035
- 10.4.4. DNA Damage Response Targeting Therapeutics Market: Distribution by Type of Molecule
- 10.4.4.1. DNA Damage Response Targeting Therapeutics Market for Biologics, Till 2035
- 10.4.4.2. DNA Damage Response Targeting Therapeutics Market for Small Molecules, Till 2035
- 10.4.5. DNA Damage Response Targeting Therapeutics Market: Distribution by Route of Administration
- 10.4.5.1. DNA Damage Response Targeting Therapeutics Market for Oral Drugs, Till 2035
- 10.4.5.2. DNA Damage Response Targeting Therapeutics Market for Intravenous Drugs, Till 2035
- 10.4.6. DNA Damage Response Targeting Therapeutics Market: Distribution by Geography
- 10.4.6.1. DNA Damage Response Targeting Therapeutics Market in the US, Till 2035
- 10.4.6.2. DNA Damage Response Targeting Therapeutics Market in Canada, Till 2035
- 10.4.6.3. DNA Damage Response Targeting Therapeutics Market in Denmark, Till 2035
- 10.4.6.4. DNA Damage Response Targeting Therapeutics Market in France, Till 2035
- 10.4.6.5. DNA Damage Response Targeting Therapeutics Market in Germany, Till 2035
- 10.4.6.6. DNA Damage Response Targeting Therapeutics Market in Italy, Till 2035
- 10.4.6.7. DNA Damage Response Targeting Therapeutics Market in Spain, Till 2035
- 10.4.6.8. DNA Damage Response Targeting Therapeutics Market in the UK, Till 2035
- 10.4.6.9. DNA Damage Response Targeting Therapeutics Market in Australia, Till 2035
- 10.4.6.10. DNA Damage Response Targeting Therapeutics Market in Singapore, Till 2035
- 10.4.6.11. DNA Damage Response Targeting Therapeutics Market in South Korea, Till 2035
- 10.4.7. Drug-wise Sales Forecast
- 10.4.7.1 Adavosertib (AZD1775, MK-1775), AstraZeneca
- 10.4.7.1.1. Target Patient Population
- 10.4.7.1.2. Sales Forecast
- 10.4.7.1.3. Net Present Value
- 10.4.7.1.4. Value Creation Analysis
- 10.4.7.2. APX3330, Apexian Pharmaceuticals
- 10.4.7.2.1. Target Patient Population
- 10.4.7.2.2. Sales Forecast
- 10.4.7.2.3. Net Present Value
- 10.4.7.2.4. Value Creation Analysis
- 10.4.7.3. ASLAN003 (LAS 186323), Aslan Pharmaceuticals
- 10.4.7.3.1. Target Patient Population
- 10.4.7.3.2. Sales Forecast
- 10.4.7.3.3. Net Present Value
- 10.4.7.3.4. Value Creation Analysis
- 10.4.7.4. CBP-501, CanBas
- 10.4.7.4.1. Target Patient Population
- 10.4.7.4.2. Sales Forecast
- 10.4.7.4.3. Net Present Value
- 10.4.7.4.4. Value Creation Analysis
- 10.4.7.5. Eprenetapopt, Aprea Therapeutics
- 10.4.7.5.1. Target Patient Population
- 10.4.7.5.2. Sales Forecast
- 10.4.7.5.3. Net Present Value
- 10.4.7.5.4. Value Creation Analysis
- 10.4.7.6. Irofulven, Allarity Therapeutics
- 10.4.7.6.1. Target Patient Population
- 10.4.7.6.2. Sales Forecast
- 10.4.7.6.3. Net Present Value
- 10.4.7.6.4. Value Creation Analysis
- 10.4.7.7. LB-100 (Lixte Biotechnology)
- 10.4.7.7.1. Target Patient Population
- 10.4.7.7.2. Sales Forecast
- 10.4.7.7.3. Net Present Value
- 10.4.7.7.4. Value Creation Analysis
- 10.4.7.8. Silmitasertib, Senhwa Biosciences
- 10.4.7.8.1. Target Patient Population
- 10.4.7.8.2. Sales Forecast
- 10.4.7.8.3. Net Present Value
- 10.4.7.8.4. Value Creation Analysis
- 10.4.7.1 Adavosertib (AZD1775, MK-1775), AstraZeneca
- 10.4.9 Concluding Remarks
- 10.4.1. DNA Damage Response Targeting Therapeutics Market: Distribution by Target Disease Indication
11. CONCLUDING REMARKS
12. APPENDIX I: TABULATED DATA
13. APPENDIX II: LIST OF COMPANIES AND ORGANIZATION
List of Tables
- Table 3.1 Key Components of DNA Repair System
- Table 3.2 Comparison of HRR and NHEJ DNA Damage Repair Pathways
- Table 3.3 DNA Damage Repair Related Inherited Mutations
- Table 4.1 DNA Damage Response Targeting Therapeutics (Clinical Pipeline): Information on Phase of Development, Target Disease Indication(s), and Therapeutic Area
- Table 4.2 DNA Damage Response Targeting Therapeutics (Clinical Pipeline): Information on Target Molecule, Type of Molecule, and Type of Therapy
- Table 4.3 DNA Damage Response Targeting Therapeutics (Clinical Pipeline): Information on Dosage Form, Route of Administration, and Special Drug Designation Awarded
- Table 4.4 DNA Damage Response Targeting Therapeutics (Preclinical Pipeline): Information on Phase of Development, Target Disease Indication(s), and Therapeutic Area
- Table 4.5 DNA Damage Response Targeting Therapeutics (Preclinical Pipeline): Information on Target Molecule, Type of Molecule, Type of Therapy and Dosage Form
- Table 4.6 DNA Damage Response Targeting Therapeutics: List of Drug Developers
- Table 6.1 DNA Damage Response Targeting Therapeutics: List of Companies Profiled
- Table 6.2 Aprea Therapeutics: Company Overview
- Table 6.3 Aprea Therapeutics: DNA Damage Response Targeting Therapeutics Portfolio
- Table 6.4 Aprea Therapeutics: Recent Developments and Future Outlook
- Table 6.5 AstraZeneca: Company Overview
- Table 6.6 AstraZeneca: DNA Damage Response Targeting Therapeutics Portfolio
- Table 6.7 AstraZeneca: Recent Developments and Future Outlook
- Table 6.8 Chordia Therapeutics: Company Overview
- Table 6.9 Chordia Therapeutics: DNA Damage Response Targeting Therapeutics Portfolio
- Table 6.10 Chordia Therapeutics: Recent Developments and Future Outlook
- Table 6.11 Mission Therapeutics: Company Overview
- Table 6.12 Mission Therapeutics: DNA Damage Response Targeting Therapeutics Portfolio
- Table 6.13 Mission Therapeutics: Recent Developments and Future Outlook
- Table 6.14 Repare Therapeutics: Company Overview
- Table 6.15 Repare Therapeutics: DNA Damage Response Targeting Therapeutics Portfolio
- Table 6.16 Repare Therapeutics: Recent Developments and Future Outlook
- Table 6.17 Senhwa Biosciences: Company Overview
- Table 6.18 Senhwa Biosciences: DNA Damage Response Targeting Therapeutics Portfolio
- Table 6.19 Senhwa Biosciences: Recent Developments and Future Outlook
- Table 8.1 List of Recent Publications, Since 2020
- Table 8.2 Publication Analysis: List of Most Valued Publications
- Table 10.1 DNA Damage Response Targeting Therapeutics: Promising Drug Candidates
- Table 10.2 DNA Damage Response Targeting Therapeutics Forecast Assumptions: Price Estimations in Key Geographies
- Table 10.3 Adavosertib (AZD1775, MK-1775), AstraZeneca: Target Patient Population
- Table 10.4 Adavosertib (AZD1775, MK-1775), AstraZeneca: Net Present Value (USDMillion)
- Table 10.5 Adavosertib (AZD1775, MK-1775), AstraZeneca: Value Creation Analysis (USDMillion)
- Table 10.6 ASLAN003 (LAS 186323, Aslan Pharmaceuticals: Target Patient Population
- Table 10.7 ASLAN003 (LAS 186323, Aslan Pharmaceuticals: Net Present Value (USDMillion)
- Table 10.8 ASLAN003 (LAS 186323, Aslan Pharmaceuticals: Value Creation Analysis (USD Million)
- Table 10.9 CBP-501, CanBas: Target Patient Population
- Table 10.10 CBP-501, CanBas: Net Present Value (USD Million)
- Table 10.11 CBP-501, CanBas: Value Creation Analysis (USD Million)
- Table 10.12 Eprenetapopt (APR-246), Aprea Therapeutics: Target Patient Population
- Table 10.13 Eprenetapopt (APR-246), Aprea Therapeutics: Net Present Value (USD Million)
- Table 10.14 Eprenetapopt, (APR-246) Aprea Therapeutics: Value Creation Analysis (USD Million)
- Table 10.15 Irofulven, Allarity Therapeutics: Target Patient Population
- Table 10.16 Irofulven, Allarity Therapeutics: Net Present Value (USD Million)
- Table 10.17 Irofulven, Allarity Therapeutics: Value Creation Analysis (USD Million)
- Table 10.18 LB-100, Lixte Biotechnology: Target Patient Population
- Table 10.19 LB-100, Lixte Biotechnology: Net Present Value (USD Million)
- Table 10.20 LB-100, Lixte Biotechnology: Value Creation Analysis (USD Million)
- Table 10.21 Sapacitabine, Cyclacel Pharmaceuticals: Target Patient Population
- Table 10.22 Sapacitabine, Cyclacel Pharmaceuticals: Net Present Value (USD Million)
- Table 10.23 Sapacitabine, Cyclacel Pharmaceuticals: Value Creation Analysis (USD Million)
- Table 10.24 Silmitasertib, Senhwa Biosciences: Target Patient Population
- Table 10.25 Silmitasertib, Senhwa Biosciences: Net Present Value (USD Million)
- Table 10.26 Silmitasertib, Senhwa Biosciences: Value Creation Analysis (USD Million)
- Table 10.27 TRC-102, TRACON Pharmaceuticals: Target Patient Population
- Table 10.28 TRC-102, TRACON Pharmaceuticals: Net Present Value (USD Million)
- Table 10.29 TRC-102, TRACON Pharmaceuticals: Value Creation Analysis (USD Million)
- Table 12.1 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development
- Table 12.2 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Target Disease Indication(s)
- Table 12.3 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development and Target Disease Indication(s)
- Table 12.4 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Therapeutic Area
- Table 12.5 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development and Therapeutic Area
- Table 12.6 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Target Molecule
- Table 12.7 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development and Target Molecule
- Table 12.8 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Type of Molecule
- Table 12.9 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Target Molecule and Type of Molecule
- Table 12.10 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Type of Therapy
- Table 12.11 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Dosage Form
- Table 12.12 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Route of Administration
- Table 12.13 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Phase of Development
- Table 12.14 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Target Disease Indication(s)
- Table 12.15 DNA Damage Response Targeting Therapeutics: (Preclinical-stage Drug Candidates) Distribution by Phase of Development and Target Disease Indication(s)
- Table 12.16 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Therapeutic Area
- Table 12.17 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Phase of Development and Therapeutic Area
- Table 12.18 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Type of Therapy
- Table 12.19 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Dosage Form
- Table 12.20 DNA Damage Response Targeting Therapeutics Developers: Distribution by Year of Establishment
- Table 12.21 DNA Damage Response Targeting Therapeutics Developers: Distribution by Company Size
- Table 12.22 DNA Damage Response Targeting Therapeutics Developers: Distribution by Location of Headquarters
- Table 12.23 Leading Developers: Distribution by Number of DNA Damage Response Targeting Therapeutics
- Table 12.24 4D Bubble Analysis: Distribution by Portfolio Strength, Target Molecule, Phase of Development and Company Size
- Table 12.25 Treemap Representation: Distribution by Therapeutic Area and Company Size
- Table 12.26 World Map Representation: Distribution by Location of Headquarters
- Table 12.27 Grid Representation: Distribution by Phase of Development, Therapeutic Area, Type of Molecule, Type of Therapy and Route of Administration
- Table 12.28 Clinical Trial Analysis: Cumulative Distribution of Trials by Registration Year, Since 2011
- Table 12.29 Clinical Trial Analysis: Distribution of Number of Patients Enrolled by Registration Year, Since 2011
- Table 12.30 Clinical Trial Analysis: Distribution of Patients Enrolled by Gender
- Table 12.31 Clinical Trial Analysis: Distribution by Trial Phase
- Table 12.32 Clinical Trial Analysis: Distribution by Year wise Distribution by Trial Phase
- Table 12.33 Clinical Trial Analysis: Distribution of Patients Enrolled by Trial Phase
- Table 12.34 Clinical Trial Analysis: Year wise Distribution of Patients Enrolled by Trial Phase
- Table 12.35 Clinical Trial Analysis: Year wise Distribution by Trial Phase and Average Completion Time
- Table 12.36 Clinical Trial Analysis: Distribution by Recruitment Status
- Table 12.37 Clinical Trial Analysis: Cumulative Year-wise Trend by Recruitment Status
- Table 12.38 Clinical Trial Analysis: Distribution by Study Design
- Table 12.39 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 12.40 Clinical Trial Analysis: Distribution by Therapeutic Area
- Table 12.41 Clinical Trial Analysis: Year wise Distribution by Most Popular Therapeutic Areas
- Table 12.42 Clinical Trial Analysis: Cumulative Year wise Trend of Patients Enrolled by Most Popular Therapeutic Areas
- Table 12.43 Clinical Trial Analysis: Distribution by Therapeutic Area and Average Completion Time
- Table 12.44 Clinical Trial Analysis: Regional Distribution of Patients Enrolled by Gender
- Table 12.45 Clinical Trial Analysis: Regional Distribution by Trial Phase and Average Completion Time
- Table 12.46 Clinical Trial Analysis: Regional Distribution by Therapeutic Area and Average Completion Time
- Table 12.47 Clinical Trial Analysis: Regional Distribution by Patients Enrolled
- Table 12.48 Clinical Trial Analysis: Regional Distribution by Average Completion Time
- Table 12.49 Clinical Trial Analysis: Year wise Distribution by Trial Phase and Average Completion Time
- Table 12.50 Leading Industry Players: Distribution by Number of Clinical Trials
- Table 12.51 Clinical Trial Analysis: Concluding Remarks
- Table 12.52 Publications Analysis: Distribution by Year of Publication
- Table 12.53 Publications Analysis: Distribution by Type of Publication
- Table 12.54 Key Journals: Distribution by Number of Publications
- Table 12.55 Publications Analysis: Distribution by Journal Impact Factor
- Table 12.56 Key Journals: Distribution by Journal Impact Factor
- Table 12.57 Key Research Hubs: Distribution by Number of Publications
- Table 12.58 Publications Analysis: Distribution by Target Molecule
- Table 12.59 Publications Analysis: Distribution by Year wise Distribution by Most Popular Target Molecules
- Table 12.60 Locations of Grant Awarding Organizations: Distribution by Number of Publications
- Table 12.61 Global DNA Damage Response Targeting Therapeutics Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.62 DNA Damage Response Targeting Therapeutics Market: Distribution by Target Disease Indication
- Table 12.63 DNA Damage Response Targeting Therapeutics Market for Acute Myeloid Leukemias, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.64 DNA Damage Response Targeting Therapeutics Market for COVID-19, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.65 DNA Damage Response Targeting Therapeutics Market for Diabetic Macular Edemas, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.66 DNA Damage Response Targeting Therapeutics Market for Mesotheliomas, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.67 DNA Damage Response Targeting Therapeutics Market for Myelodysplastic Syndromes, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.68 DNA Damage Response Targeting Therapeutics Market for Non-Squamous Non-Small Cell Lung Cancers, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.69 DNA Damage Response Targeting Therapeutics Market for Prostate Cancers, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.70 DNA Damage Response Targeting Therapeutics Market for Uterine Serous Carcinomas, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.71 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Therapeutic Area
- Table 12.72 DNA Damage Response Targeting Therapeutics Market for Hematological Malignancies, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.73 DNA Damage Response Targeting Therapeutics Market for Solid Tumors, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.74 DNA Damage Response Targeting Therapeutics Market for Other Disorders, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.75 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Target Molecule
- Table 12.76 Damage Response Targeting Therapeutics Market for APE1/Ref-1, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.77 Damage Response Targeting Therapeutics Market for Casein Kinase 2, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.78 Damage Response Targeting Therapeutics Market for CHK-1, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.79 Damage Response Targeting Therapeutics Market for C-Tak, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.80 Damage Response Targeting Therapeutics Market for DHODH, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.81 Damage Response Targeting Therapeutics Market for MAPKAPK2, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.82 Damage Response Targeting Therapeutics Market for p53, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.83 Damage Response Targeting Therapeutics Market for Protein Phosphatase 2A Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.84 Damage Response Targeting Therapeutics Market for WEE 1, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.85 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Type of Molecule
- Table 12.86 DNA Damage Response Targeting Therapeutics Market for Biologics, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.87 DNA Damage Response Targeting Therapeutics Market for Small Molecules, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.88 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Route of Administration
- Table 12.89 DNA Damage Response Targeting Therapeutics Market for Oral Drugs, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.90 DNA Damage Response Targeting Therapeutics Market for Intravenous Drugs, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.91 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Geography
- Table 12.92 DNA Damage Response Targeting Therapeutics Market in the US, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.93 DNA Damage Response Targeting Therapeutics Market in the Canada, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.94 DNA Damage Response Targeting Therapeutics Market in the Denmark, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.95 DNA Damage Response Targeting Therapeutics Market in France, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.96 DNA Damage Response Targeting Therapeutics Market in Germany, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.97 DNA Damage Response Targeting Therapeutics Market in Italy, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.98 DNA Damage Response Targeting Therapeutics Market in Spain, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.99 DNA Damage Response Targeting Therapeutics Market in the UK, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.100 DNA Damage Response Targeting Therapeutics Market in Australia, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.101 DNA Damage Response Targeting Therapeutics Market in Singapore, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.102 DNA Damage Response Targeting Therapeutics Market in South Korea, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.103 Adavosertib (AZD1775, MK-1775), AstraZeneca: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios (USD Million)
- Table 12.104 APX3330, Apexian Pharmaceuticals: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.105 ASLAN003 (LAS 186323), Aslan Pharmaceuticals: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.106 CBP-501, CanBas: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.107 Eprenetapopt, Aprea Therapeutics: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.108 Irofulven, Allarity Therapeutics: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.109 LB-100, Lixte Biotechnology: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.110 Silmitasertib, Senhwa Biosciences: Sales Forecast, till 2030, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Table 12.111 Global DNA Damage Response Targeting Therapeutics Market, Conservative, Base and Optimistic Scenarios, Till 2035: Conservative, Base and Optimistic Scenarios (USD Million)
List of Figures
- Figure 3.1 Type of DNA Damage
- Figure 3.2 Types of Causative Agents for DNA Damage
- Figure 3.3 DNA Damage Causative Agents and Associated Repair System
- Figure 3.4 DNA Damage Response System
- Figure 3.5 Direct DNA Damage Repair Systems
- Figure 3.6 Key Steps Involved in Base Excision Repair Pathway
- Figure 3.7 Key Steps Involved in Nucleotide Excision Repair Pathway
- Figure 3.8 Key Steps Involved in Mismatch Repair Pathway
- Figure 3.9 Key Steps Involved in Homologous Recombination Repair Pathway
- Figure 3.10 Key Steps Involved in Non-Homologous Repair Pathway
- Figure 4.1 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development
- Figure 4.2 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Target Disease Indication(s)
- Figure 4.3 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development and Target Disease Indication(s)
- Figure 4.4 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Therapeutic Area
- Figure 4.5 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution Phase of Development and Therapeutic Area
- Figure 4.6 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Target Molecule
- Figure 4.7 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Phase of Development and Target Molecule
- Figure 4.8 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Type of Molecule
- Figure 4.9 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Target Molecule and Type of Molecule
- Figure 4.10 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Type of Therapy
- Figure 4.11 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Dosage Form
- Figure 4.12 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Route of Administration
- Figure 4.13 DNA Damage Response Targeting Therapeutics (Clinical-stage Drug Candidates): Distribution by Special Drug Designation Awarded
- Figure 4.14 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Phase of Development
- Figure 4.15 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Target Disease Indication(s)
- Figure 4.16 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Phase of Development and Target Disease Indication(s)
- Figure 4.17 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Therapeutic Area
- Figure 4.18 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Phase of Development and Therapeutic Area
- Figure 4.19 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Type of Therapy
- Figure 4.20 DNA Damage Response Targeting Therapeutics (Preclinical-stage Drug Candidates): Distribution by Dosage Form
- Figure 4.21 DNA Damage Response Targeting Therapeutics Developers: Distribution by Year of Establishment
- Figure 4.22 DNA Damage Response Targeting Therapeutics Developers: Distribution by Company Size
- Figure 4.23 DNA Damage Response Targeting Therapeutics Developers: Distribution by Location of Headquarters
- Figure 4.24 Leading Developers: Distribution by Number of DNA Damage Response Targeting Therapeutics
- Figure 5.1 4D Bubble Representation: Distribution by Portfolio Strength, Target Molecule, Phase of Development and Company Size
- Figure 5.2 Treemap Representation: Distribution by Therapeutic Area and Company Size
- Figure 5.3 World Map Representation: Distribution by Location of Headquarters
- Figure 5.4 Grid Representation: Distribution by Phase of Development, Therapeutic Area, Type of Molecule, Type of Therapy and Route of Administration
- Figure 7.1 Clinical Trial Analysis: Scope and Methodology
- Figure 7.2 Clinical Trial Analysis: Cumulative Distribution of Trials by Registration Year, Since 2011
- Figure 7.3 Clinical Trial Analysis: Distribution of Number of Patients Enrolled by Registration Year, Since 2011
- Figure 7.4 Clinical Trial Analysis: Distribution of Patients Enrolled by Gender
- Figure 7.5 Clinical Trial Analysis: Distribution by Trial Phase
- Figure 7.6 Clinical Trial Analysis: Year wise Distribution by Trial Phase
- Figure 7.7 Clinical Trial Analysis: Distribution of Patients Enrolled by Trial Phase
- Figure 7.8 Clinical Trial Analysis: Year wise Distribution of Patients Enrolled by Trial Phase
- Figure 7.9 Clinical Trial Analysis: Year wise Distribution by Trial Phase and Average Completion Time
- Figure 7.10 Clinical Trial Analysis: Distribution by Recruitment Status
- Figure 7.11 Clinical Trial Analysis: Cumulative Year wise Distribution by Recruitment Status
- Figure 7.12 Clinical Trial Analysis: Distribution by Study Design
- Figure 7.13 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Figure 7.14 Clinical Trial Analysis: Distribution by Therapeutic Area
- Figure 7.15 Clinical Trial Analysis: Year wise Distribution by Most Popular Therapeutic Areas
- Figure 7.16 Clinical Trial Analysis: Cumulative Year wise Trend of Patients Enrolled by Most Popular Therapeutic Areas
- Figure 7.17 Clinical Trial Analysis: Distribution by Therapeutic Area and Average Completion Time
- Figure 7.18 Clinical Trial Analysis: Regional Distribution
- Figure 7.19 Clinical Trial Analysis: Regional Distribution by Trial Phase and Average Completion Time
- Figure 7.20 Clinical Trial Analysis: Regional Distribution by Therapeutic Area and Average Completion Time
- Figure 7.21 Clinical Trial Analysis: Regional Distribution by Patients Enrolled
- Figure 7.22 Clinical Trial Analysis: Regional Distribution by Average Completion Time
- Figure 7.23 Clinical Trial Analysis: Year wise Distribution by Trial Phase and Average Completion Time
- Figure 7.24 Leading Industry Players: Distribution by Number of Clinical Trials
- Figure 7.25 Clinical Trial Analysis: Concluding Remarks
- Figure 8.1 Publications Analysis: Methodology
- Figure 8.2 Publications Analysis: Distribution by Year of Publication
- Figure 8.3 Publications Analysis: Distribution by Type of Publication
- Figure 8.4 Publications Analysis: Emerging Focus Areas
- Figure 8.5 Key Journals: Distribution by Number of Publications
- Figure 8.6 Publications Analysis: Distribution by Journal Impact Factor
- Figure 8.7 Key Journals: Distribution by Journal Impact Factor
- Figure 8.8 Key Research Hubs: Distribution by Number of Publications
- Figure 8.9 Publications Analysis: Distribution by Target Molecule
- Figure 8.10 Publications Analysis: Year wise Distribution by Most Popular Target Molecules
- Figure 8.11 Locations of Grant Awarding Organizations: Distribution by Number of Publications
- Figure 8.12 Publications Analysis: Multivariate Benchmark Analysis
- Figure 9.1 Key Parameters Impacting Drug Pricing and Adoption
- Figure 9.2 Adavosertib (AZD1775) (MK-1775)
- Figure 9.3 APX3330
- Figure 9.4 ASLAN003
- Figure 9.5 CBP-501
- Figure 9.5 Eprenetapopt
- Figure 9.6 Irofulven
- Figure 9.7 LB-100
- Figure 9.8 Silmitasertib
- Figure 9.9 TRC-102
- Figure 10.1 Global DNA Damage Response Targeting Therapeutics Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Million)
- Figure 10.2 DNA Damage Response Targeting Therapeutics Market: Distribution by Target Disease Indication
- Figure 10.3 DNA Damage Response Targeting Therapeutics Market for Acute Myeloid Leukemias, Till 2035 (USD Million)
- Figure 10.4 DNA Damage Response Targeting Therapeutics Market for COVID-19, Till 2035 (USD Million)
- Figure 10.5 DNA Damage Response Targeting Therapeutics Market for Diabetic Macular Edemas, Till 2035 (USD Million)
- Figure 10.6 DNA Damage Response Targeting Therapeutics Market for Mesotheliomas, Till 2035 (USD Million)
- Figure 10.7 DNA Damage Response Targeting Therapeutics Market for Myelodysplastic Syndromes, Till 2035 (USD Million)
- Figure 10.8 DNA Damage Response Targeting Therapeutics Market for Non-Squamous Non-Small Cell Lung Cancers, Till 2035 (USD Million)
- Figure 10.9 DNA Damage Response Targeting Therapeutics Market for Prostate Cancer, Till 2035 (USD Million)
- Figure 10.10 DNA Damage Response Targeting Therapeutics Market for Uterine Serous Carcinomas, Till 2035 (USD Million)
- Figure 10.11 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Therapeutic Area
- Figure 10.12 DNA Damage Response Targeting Therapeutics Market for Hematological Malignancies, Till 2035 (USD Million)
- Figure 10.13 DNA Damage Response Targeting Therapeutics Market for Solid Tumors, Till 2035 (USD Million)
- Figure 10.14 DNA Damage Response Targeting Therapeutics Market for Other Disorders, Till 2035 (USD Million)
- Figure 10.15 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Target Molecule
- Figure 10.16 DNA Damage Response Targeting Therapeutics Market for APE1/Ref-1, Till 2035 (USD Million)
- Figure 10.17 DNA Damage Response Targeting Therapeutics Market for Casein Kinase 2, Till 2035 (USD Million)
- Figure 10.18 DNA Damage Response Targeting Therapeutics Market for CHK-1, Till 2035 (USD Million)
- Figure 10.19 DNA Damage Response Targeting Therapeutics Market for C-Tak, Till 2035 (USD Million)
- Figure 10.20 DNA Damage Response Targeting Therapeutics Market for DHODH, Till 2035 (USD Million)
- Figure 10.21 DNA Damage Response Targeting Therapeutics Market for MAPKAPK2, Till 2035 (USD Million)
- Figure 10.22 DNA Damage Response Targeting Therapeutics Market for p53, Till 2035 (USD Million)
- Figure 10.23 DNA Damage Response Targeting Therapeutics Market for Protein Phosphatase 2A, Till 2035 (USD Million)
- Figure 10.24 DNA Damage Response Targeting Therapeutics Market for WEE 1, Till 2035 (USD Million)
- Figure 10.25 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Type of Molecule
- Figure 10.26 DNA Damage Response Targeting Therapeutics Market for Biologics, Till 2035 (USD Million)
- Figure 10.27 DNA Damage Response Targeting Therapeutics Market for Small Molecules, Till 2035 (USD Million)
- Figure 10.28 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Route of Administration
- Figure 10.29 DNA Damage Response Targeting Therapeutics Market for Oral Drugs, Till 2035 (USD Million)
- Figure 10.30 DNA Damage Response Targeting Therapeutics Market for Intravenous Drugs, Till 2035 (USD Million)
- Figure 10.31 Global DNA Damage Response Targeting Therapeutics Market: Distribution by Geography
- Figure 10.32 DNA Damage Response Targeting Therapeutics Market in the US, Till 2035 (USD Million)
- Figure 10.33 DNA Damage Response Targeting Therapeutics Market in Canada, Till 2035 (USD Million)
- Figure 10.34 DNA Damage Response Targeting Therapeutics Market in Denmark, Till 2035 (USD Million)
- Figure 10.35 DNA Damage Response Targeting Therapeutics Market in France, Till 2035 (USD Million)
- Figure 10.36 DNA Damage Response Targeting Therapeutics Market in Germany, Till 2035 (USD Million)
- Figure 10.37 DNA Damage Response Targeting Therapeutics Market in Italy, Till 2035 (USD Million)
- Figure 10.38 DNA Damage Response Targeting Therapeutics Market in Spain, Till 2035 (USD Million)
- Figure 10.39 DNA Damage Response Targeting Therapeutics Market in the UK, Till 2035 (USD Million)
- Figure 10.40 DNA Damage Response Targeting Therapeutics Market in Australia, Till 2035 (USD Million)
- Figure 10.41 DNA Damage Response Targeting Therapeutics Market in Singapore, Till 2035 (USD Million)
- Figure 10.42 DNA Damage Response Targeting Therapeutics Market in South Korea, Till 2035 (USD Million)
- Figure 10.43 Adavosertib (AZD1775, MK-1775), AstraZeneca: Sales Forecast, till 2030 (USD Million)
- Figure 10.44 APX3330, Apexian Pharmaceuticals: Sales Forecast, till 2030 (USD Million)
- Figure 10.45 ASLAN003 (LAS 186323), Aslan Pharmaceuticals: Sales Forecast, till 2030 (USD Million)
- Figure 10.46 CBP-501, CanBas: Sales Forecast, till 2030 (USD Million)
- Figure 10.47 Eprenetapopt (APR-246), Aprea Therapeutics: Sales Forecast, till 2030 (USD Million)
- Figure 10.48 Irofulven, Allarity Therapeutics: Sales Forecast, till 2030 (USD Million)
- Figure 10.49 LB-100, Lixte Biotechnology: Sales Forecast, till 2030 (USD Million)
- Figure 10.50 Silmitasertib, Senhwa Biosciences: Sales Forecast, till 2030 (USD Million)
- Figure 10.51 Global DNA Damage Response Targeting Therapeutics Market, Till 2035: Conservative, Base and Optimistic Scenarios, 2021, 2025 and 2030 (USD Million)
- Figure 11.1 Concluding Remarks: Market Landscape
- Figure 11.2 Concluding Remarks: Clinical Trial Analysis
- Figure 11.3 Concluding Remarks: Publications
- Figure 11.4 Concluding Remarks: Key Parameters Impacting Drug Pricing and Adoption
- Figure 11.5 Concluding Remarks: Market Forecast










