 |
市場調查報告書
商品編碼
1762532
醫療器材合約製造市場:產業趨勢及全球預測 - 依應用、設備類別、治療領域和重點地區Medical Device Contract Manufacturing Market: Industry Trends and Global Forecasts - Distribution by Application Area, Device Class, Target Therapeutic Area, Key Geographical Regions |
||||||
醫療器材合約製造市場:概覽
今年全球醫療器材合約製造市場規模達 788億美元。預計預測期內,該市場的年複合成長率將達到 8.2%。
市場區隔包括根據以下參數進行的市場規模和機會分析:
應用領域
- 治療器械
- 診斷器械
- 藥物輸送器械
- 其他器械
器械類別
- I類
- II類
- III類
目標治療領域
- 心血管疾病
- 中樞神經系統疾病
- 代謝性疾病
- 腫瘤疾病
- 眼科
- 骨科
- 疼痛障礙
- 呼吸系統疾病
- 其他
主要地區
- 北美洲(美國)
- 歐洲(義大利、德國、法國、西班牙、英國及世界其他地區)
- 亞太地區(中國、日本、印度)
- 其他地區
醫療器材合約製造市場:成長與趨勢
在過去的50年中,醫療器械行業取得了多項顯著進展,包括微創手術器械的引進以及起搏器等植入式器械的興起。此外,假體等生物醫學器械的使用也隨著時間的推移顯著增加,每年有數百萬人接受手術和植入。此外,心臟去顫器、人工髖關節和膝關節、隱形眼鏡、心臟節律器等是最常見的植入式醫療器械,而固定裝置和人工關節約佔所有醫療器械的44%。這顯示醫療保健領域對醫療器材的需求成長。然而,大多數開發商缺乏足夠的資源或必要的專業知識來製造醫療器材及相關零件。
隨著醫療器材合約研究組織(CRO)的興起,擁有醫療器材專業知識的合約製造組織(CMO)領域也在穩步擴張。值得注意的是,自2000年以來,已有超過65家CMO成立。此外,根據世界衛生組織(WHO)的報告,全球約有200萬種醫療器械,分為7,000多個通用器械類別。值得注意的是,CMO以其顯著的成本效益、先進的現代化基礎設施、大規模的生產能力以及更快的上市時間而聞名。因此,醫療器材合約製造服務提供者的機會穩步成長。
醫療器材合約製造市場:關鍵洞察
本報告深入探討了醫療器材合約製造市場的現狀,並識別了行業內的潛在成長機會。主要調查結果包括:
- 新興技術和平台已成為眾多中小型醫療器材公司成立和成功的主要驅動力之一。
- 監管合規和審批的複雜性、不斷上漲的製造成本以及多樣化的技術,促使申辦方尋求能夠支持其器械整個生命週期的製造合作夥伴。
- 全球超過 275 家公司擁有為各種醫療器材(包括治療和診斷產品)提供合約製造服務所需的專業知識和授權。
- 市場格局分散,既有老牌企業,也有新進者。目前,大多數醫療器材製造工廠都位於已開發地區。
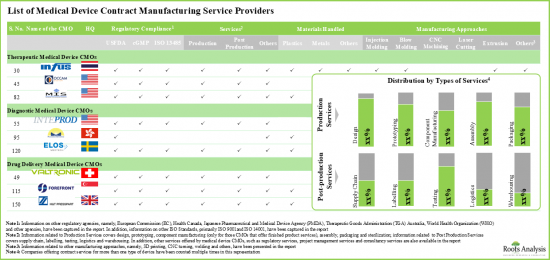
- 醫療器材合約製造企業(CMO)積極調整運營,以符合各全球和區域監管機構制定的標準。
- 植入物已成為最突出的細分市場之一,其收購活動相對較多。此類收購背後的主要價值驅動因素包括產能擴張和地理整合。
- 在過去六年中,各類申辦方已註冊了超過 9,600 項評估各種醫療器材的臨床試驗,涵蓋了廣泛的治療領域。
- 在該領域運營的公司穩步擴展其能力,以增強各自的服務組合,並遵守不斷變化的行業基準。
- 在快速成長的需求推動下,預計醫療器材合約製造市場在各個治療領域和地區的成品年成長率將超過 8.2%。
- 目前,現有的CMO 佔據了很大的佔有率,但從長遠來看,預計不同類型的公司、不同的器械類別和不同的應用領域將帶來多樣化的機會。
醫療器材合約製造市場:關鍵細分市場
市場依應用細分為治療醫療器材、診斷醫療器材、給藥醫療器材和其他器材。目前,由於胰島素幫浦和輸液幫浦等治療設備的需求不斷成長,治療醫療器材領域在全球醫療器材合約製造市場中佔據最大佔有率。此外,預計在預測期內,藥物傳輸設備領域的醫療器材合約製造市場將呈現最高的市場成長。
依器械類別,市場分為I類、II類及III類。目前,II類器械在全球醫療器材合約製造市場中佔據最大佔有率。此外,由於II類器材具有易於客製化和產品種類豐富等諸多優勢,因此預計在預測期內,II類器材的市場將以更高的年複合成長率成長。
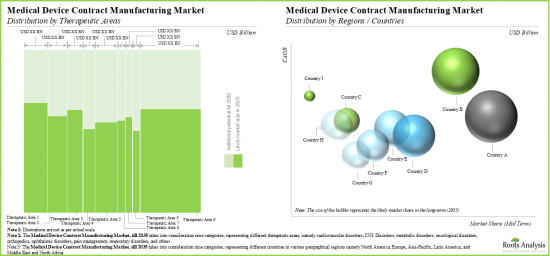
根據涵蓋的治療領域,市場細分為心血管疾病、中樞神經系統疾病、代謝性疾病、腫瘤疾病、骨科疾病、眼科疾病、疼痛疾病、呼吸系統疾病等。目前,骨科疾病領域在全球醫療器材合約製造市場中佔最大佔有率。然而,由於肥胖、胰島素阻抗、第1型糖尿病和高血壓等代謝性疾病的盛行率不斷上升,預計代謝性疾病領域在預測期內的年複合成長率將更高。
依主要地區劃分,市場分為北美、歐洲、亞太地區及世界其他地區。目前,北美在全球醫療器材合約製造市場中佔據主導地位,佔據最大的收入佔有率。此外,亞太地區市場未來很可能會以更高的年複合成長率成長。
醫療器材合約製造市場參與者
- Cirtec Medical
- Creganna Medical
- DynaFlex Technologies
- Europlaz Technologies
- I-Tek Medical Technologies
- Interplex
- Keystone Solutions Group
- Modern Medical
- Oscor
- Riverside Medical Packaging
- SMC
- Synecco
- Suzhou Jenitek
- Stellartech Research
- Trelleborg Sealing Solutions
目錄
第1章 簡介
第2章 執行摘要
第3章 簡介
- 章節概述
- 醫療器材:概述
- 醫療器材製造
- 醫療器材CMO歷史年表
- 醫療器材CMO提供的服務
- 醫療器材CMO提供的優勢
- 外包給CMO的風險
- 關鍵考慮因素
- 結論
第4章 醫療器材監管格局
- 章節概述
- 醫療器材通用監管指南
- 北美監理格局
- 歐洲監管格局
- 亞太監管格局
- 區域監理環境比較
第5章 市場概述:治療器材
- 章節概述
- 為治療器材提供服務的醫療器材CMO設備
第6章 市場概覽:診斷設備
- 章節概覽
- 提供診斷設備服務的醫療器材合約生產企業(CMO)
第7章 市場概覽:輸送系統及其他
- 章節概覽
- 導管
- 藥物傳輸設備
- 其他設備
第8章 基準分析
- 章節概覽
- 基準分析:研究方法
- 依地區劃分的基準分析
- 結論
第9章 供應鏈分析
- 章節概覽
- 醫療器材供應鏈概覽
- 影響醫療器材供應的因素供應鏈
- 關鍵績效指標
- 供應鏈改善策略
- 供應鏈最佳化的有效性
- 成本分析
第10章 公司簡介
- 章節概述
- 總部位於北美的CMO
- Cirtec Medical
- DynaFlex Technologies
- I-Tek Medical Technologies
- Keystone Solutions Group
- Oscor
- SMC
- Stellartech Research
- 總部位於歐洲的CMO
- Creganna Medical
- Europlaz Technologies
- Riverside Medical Packaging
- Synecco
- Trelleborg Sealing Solutions
- 總部位於亞洲的CMO
- Interplex
- Modern Medical
- Providence Enterprise
- Suzhou JenitekJenitek
第11章 臨床試驗分析
- 章節概述
- 研究範圍與研究方法
- 臨床試驗分析:研究標題詞雲
- 臨床試驗分析
- 入組受試者分析
- 重點治療領域分析
第12章 併購
第13章 案例研究:離岸醫療器材合約製造
- 章節概述
- 新興市場:定義與關鍵區域
- 新興市場在工業製造中的作用
- 新興市場的醫療器材製造
- 致力於新興市場醫療器材製造的跨國公司市場
- 新興市場醫療器材製造的商業模式
- 醫療器材產業創新面臨的關鍵挑戰
- 在新興市場建立創新中心的優勢
- 未來展望
第14章 市場預測
- 章節概述
- 預測研究方法與關鍵假設
- 2035年醫療器材合約製造市場整體狀況
- 醫療器材合約製造市場:區域分佈(~2035年)
第15章 SWOT分析
第16章 結論
第17章 調查/訪談紀錄
第18章 附錄1:表格資料
第19章 附錄2:公司與組織清單
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: OVERVIEW
As per Roots Analysis, the global medical device contract manufacturing market valued at USD 78.8 billion in the current year is anticipated to grow at a CAGR of 8.2% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Application Areas
- Therapeutic Medical Devices
- Diagnostic Medical Devices
- Drug Delivery Medical Devices
- Other Devices
Device Class
- Class I
- Class II
- Class III
Target Therapeutic Area
- Cardiovascular Disorders
- CNS Disorders
- Metabolic Disorders
- Oncological Disorders
- Ophthalmic Disorders
- Orthopedic Disorders
- Pain Disorders
- Respiratory Disorders
- Others
Key Geographical Regions
- North America (US)
- Europe (Italy, Germany, France, Spain, UK, Rest of the Europe)
- Asia-Pacific (China, Japan, India)
- Rest of the World
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: GROWTH AND TRENDS
Over the past 50 years, the medical device industry has seen several notable developments, such as the introduction of minimally invasive surgical instruments and the rise of implantable devices like pacemakers. Additionally, the use of biomedical devices like prostheses has increased substantially over time owing to the fact that each year, millions of individuals undergo surgeries and implantations. Further, cardioverter defibrillators, prosthetic hips and knees, contact lenses, and cardiac pacemakers are some of the most commonly implanted medical devices, while fixation devices and artificial joints account for about 44% of all medical devices. This is indicative of the growing demand for medical devices in the healthcare domain. However, most developers lack adequate resources and the necessary expertise to manufacture medical devices and related components.

Given the rise of medical device CROs, the opportunity for CMOs with expertise in medical devices is also steadily rising. It is worth highlighting that, since 2000, more than 65 CMOs have been established. Further, the World Health Organization reports approximately 2 million unique medical devices worldwide, classified into more than 7,000 generic device categories. Notably, CMOs are known to offer significant cost-benefits, access to sophisticated / up-to-date infrastructure, large production capacities and reduction in time-to-market. As a result, the opportunity for medical device contract manufacturing service providers is steadily increasing.
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: KEY INSIGHTS
The report delves into the current state of the medical device contract manufacturing market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Novel technologies and platforms have emerged as one of the primary factors responsible for the establishment and success of a number of small and mid-sized medical device companies.
- Owing to complexities associated with regulatory compliance and approvals, increasing manufacturing costs and varying technologies, sponsors seek a manufacturing partner that can support the entire lifecycle of the device.
- Over 275 companies worldwide have the necessary expertise and authorization to offer contract manufacturing services for various types of medical devices, including therapeutic and diagnostic products
- The market landscape is fragmented, featuring the presence of both established players and new entrants; presently, most medical device manufacturing facilities are located in the developed geographies.

- Medical device CMOs are actively engaged in aligning their operations to comply with the standards established by various global and regional regulatory bodies.
- Implants have emerged as one of the most prominent segments for which the acquisition activity is relatively higher; key value drivers behind such acquisitions include capability addition and geographical consolidation.
- In the last six years, over 9,600 trials evaluating various medical devices have been registered by different types of sponsors covering a wide range of therapeutic areas.
- Companies involved in this domain are steadily expanding their capabilities in order to enhance their respective service portfolios and comply with evolving industry benchmarks.
- Driven by a rapidly growing demand, medical device contract services market is anticipated to grow at annualized rate of over 8.2% for a variety of therapeutic areas and geographies for finished products.

- Established CMOs currently occupy a major share; in the long term, the opportunity is anticipated to be better distributed across different types of companies, device classes and application areas.
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: KEY SEGMENTS
Therapeutic Medical Devices Segment holds the Largest Share of the Medical Device Contract Manufacturing Market
Based on the application areas, the market is segmented into therapeutic medical devices, diagnostic medical devices, drug delivery medical devices and other devices. At present, the therapeutic medical devices segment holds the maximum share of the global medical device contract manufacturing market owing to the increasing demand for therapeutic devices such as insulin pumps and infusion pumps. Further, the medical device contract manufacturing market for drug delivery devices segment is expected to show the highest market growth potential during the forecast period.
By Device Class, Class II is the Fastest Growing Segment of the Global Medical Device Contract Manufacturing Market
Based on the device class, the market is segmented into Class I, Class II and Class III. At present, the class II segment holds the maximum share of the global medical device contract manufacturing market. Further, owing to the various benefits offered by class II devices, such as ease of customization and diverse product range, the market for class II segment is expected to grow at a higher CAGR during the forecast period.
By Target Therapeutic Area, Orthopedic Disorders Segment Accounts for the Largest Share of the Global Medical Device Contract Manufacturing Market
Based on the target therapeutic area, the market is segmented into cardiovascular disorders, CNS disorders, metabolic disorders, oncological disorders, orthopedic disorders, ophthalmic disorders, pain disorders, respiratory disorders, and others. Currently, the orthopedic disorders segment captures the highest proportion of the global medical device contract manufacturing market. However, the metabolic disorders segment is expected to grow at a higher CAGR during the forecast period owing to the growing prevalence of metabolic disorders such as obesity, insulin resistance, Type 1 diabetes and hypertension.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the World. Currently, North America dominates the global medical device contract manufacturing market and accounts for the largest revenue share. Further, the market in Asia-Pacific is likely to grow at a higher CAGR in the coming future.
Example Players in the Medical Device Contract Manufacturing Market
- Cirtec Medical
- Creganna Medical
- DynaFlex Technologies
- Europlaz Technologies
- I-Tek Medical Technologies
- Interplex
- Keystone Solutions Group
- Modern Medical
- Oscor
- Riverside Medical Packaging
- SMC
- Synecco
- Suzhou Jenitek
- Stellartech Research
- Trelleborg Sealing Solutions
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global medical device contract manufacturing market, focusing on key market segments, including [A] application areas, [B] device class, [C] target therapeutic area and [D] key geographical regions.
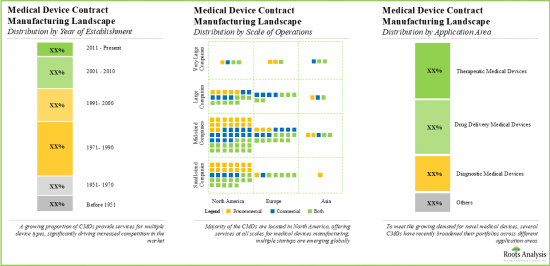
- Market Landscape: A comprehensive evaluation of the medical device contract manufacturers, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] manufacturing facilities, [D] geographical location, [E] type of device manufactured, [F] the scale of operation and [G] types of services offered.
- Regulatory Landscape for Medical Devices: A comprehensive discussion of the various guidelines established by major regulatory bodies for medical device approval across different countries. Additionally, the section includes a multi-dimensional bubble analysis, focusing on a comparison of the contemporary regulatory scenario in key geographies across the globe.
- Benchmark Analysis: A comprehensive benchmark analysis emphasizing the primary focus areas of small, mid-sized, and large companies, comparing their current capabilities within and across peer groups, and offering stakeholders insights to achieve a competitive advantage in the industry.
- Supply Chain Analysis: A detailed analysis of medical device supply chain, highlighting the role of CMOs engaged in the medical device contract manufacturing market and analysis based on services offered, such as [A] supply chain management services, [B] logistics services, [C] shipping services, and [D] warehousing services.
- Company Profiles: In-depth profiles of key players that specialize in providing services for both pre-commercial and commercial scale manufacturing of medical devices, focusing on [A] overview of the company, [B] service portfolio, [C] manufacturing facilities and [D] recent developments and an informed future outlook.
- Clinical Trial Analysis: An insightful analysis of clinical trials related to medical devices, based on several parameters, such as [A] number of registered trials, [B] current status of trials, [C] phase of development, [D] type of sponsor, [E] therapeutic area(s), [F] target disease indication(s), and [G] number of patients enrolled.
- Mergers and Acquisitions: An in-depth analysis of mergers and acquisitions undertaken in the medical device contract manufacturing market highlighting the number of companies acquired in recent years. Further, in depth-analysis of the key value drivers for these mergers and acquisitions was performed. In addition, the analysis features an ownership change matrix, providing a summary of the involvement of private and public sector entities in the market.
- SWOT Analysis: An analysis of industry affiliated trends, opportunities and challenges, which are likely to impact the evolution of antibody contract manufacturing market; it includes a Harvey ball analysis, assessing the relative impact of each SWOT parameter on industry dynamics.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Medical Devices: An Overview
- 3.2.1. History of Medical Devices
- 3.2.2. Classification of Medical Devices
- 3.3. Medical Device Manufacturing
- 3.3.1. Challenges Associated with Medical Device Manufacturing
- 3.3.2. Role of CMOs in Medical Device Manufacturing
- 3.3.3. Role of Automation in Medical Device Manufacturing Process
- 3.4. Historical Timeline for Medical Device CMOs
- 3.5. Services Offered by Medical Device CMOs
- 3.6. Advantages Offered by Medical Device CMOs
- 3.7. Risks associated with Outsourcing to CMOs
- 3.8. Key Considerations
- 3.9. Concluding Remarks
4. REGULATORY LANDSCAPE FOR MEDICAL DEVICES
- 4.1. Chapter Overview
- 4.2. General Regulatory Guidelines for Medical Devices
- 4.3. Regulatory Landscape in North America
- 4.3.1. The US Scenario
- 4.3.1.1. Regulatory Authority
- 4.3.1.2. Review / Approval Process
- 4.3.2. The Canadian Scenario
- 4.3.2.1. Regulatory Authority
- 4.3.2.2. Review / Approval Process
- 4.3.1. The US Scenario
- 4.4. Regulatory Landscape in Europe
- 4.4.1. Overall Scenario
- 4.4.1.1. Regulatory Authority
- 4.4.1.2. Review / Approval Process
- 4.4.2. Case Study: Brexit -The UK Scenario
- 4.4.1. Overall Scenario
- 4.5. Regulatory Landscape in Asia-Pacific
- 4.5.1. The Chinese Scenario
- 4.5.1.1. Regulatory Authority
- 4.5.1.2. Review / Approval Process
- 4.5.2. The Japanese Scenario
- 4.5.2.1. Regulatory Authority
- 4.5.2.2. Review / Approval Process
- 4.5.3. The Australian Scenario
- 4.5.3.1. Regulatory Authority
- 4.5.3.2. Review / Approval Process
- 4.5.1. The Chinese Scenario
- 4.6. Comparison of Regional Regulatory Environment
5. MARKET OVERVIEW: THERAPEUTIC DEVICES
- 5.1. Chapter Overview
- 5.2. Medical Device CMOs Offering Services for Therapeutic Devices
- 5.2.1. Analysis by Year of Establishment
- 5.2.2. Analysis by Size of Employee Base
- 5.2.3. Analysis by Location of Headquarters
- 5.2.4. Analysis by Location of Manufacturing Facility
- 5.2.5. Analysis by Regulatory Certifications / Accreditations
- 5.2.6. Analysis by Production Services Offered
- 5.2.7. Analysis by Post-production Services Offered
- 5.2.8. Analysis by Other Services Offered
- 5.2.9. Analysis by Device Class
- 5.2.10. Analysis by Type of Material(s) Handled
- 5.2.11. Analysis by Scale of Operation
- 5.2.12. Leading Players
6. MARKET OVERVIEW: DIAGNOSTIC DEVICES
- 6.1. Chapter Overview
- 6.2. Medical Device CMOs Offering Services for Diagnostic Devices
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Size of Employee Base
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by Location of Manufacturing Facility
- 6.2.5. Analysis by Regulatory Certifications / Accreditations
- 6.2.6. Analysis by Production Services Offered
- 6.2.7. Analysis by Post-Production Services Offered
- 6.2.8. Analysis by Other Services Offered
- 6.2.9. Analysis by Device Class
- 6.2.10. Analysis by Type of Material(s) Handled
- 6.2.11. Analysis by Scale of Operation
- 6.2.12. Leading Players
7. MARKET OVERVIEW: DELIVERY SYSTEMS AND OTHERS
- 7.1. Chapter Overview
- 7.2. Catheters
- 7.2.1. Medical Device CMOs Offering Services for Catheters
- 7.2.2. Analysis by Year of Establishment
- 7.2.3. Analysis by Size of Employee Base
- 7.2.4. Analysis by Location of Headquarters
- 7.2.5. Analysis by Location of Manufacturing Facility
- 7.2.6. Analysis by Regulatory Certifications / Accreditations
- 7.2.7. Analysis by Production Services Offered
- 7.2.8. Analysis by Post-Production Services Offered
- 7.2.9. Analysis by Other Services Offered
- 7.2.10. Analysis by Device Class
- 7.2.11. Analysis by Type of Material(s) Handled
- 7.2.12. Analysis by Scale of Operation
- 7.2.13. Leading Players
- 7.3. Drug Delivery Devices
- 7.3.1. Medical Device CMOs Offering Services for Drug Delivery Devices
- 7.3.2. Analysis by Year of Establishment
- 7.3.3. Analysis by Size of Employee Base
- 7.3.4. Analysis by Location of Headquarters
- 7.3.5. Analysis by Location of Manufacturing Facility
- 7.3.6. Analysis by Regulatory Certifications / Accreditations
- 7.3.7. Analysis by Production Services Offered
- 7.3.8. Analysis by Post-Production Services Offered
- 7.3.9. Analysis by Other Services Offered
- 7.3.10. Analysis by Device Class
- 7.3.11. Analysis by Type of Material(s) Handled
- 7.3.12. Analysis by Scale of Operation
- 7.3.13. Leading Players
- 7.4. Other Devices
- 7.4.1. Medical Device CMOs Offering Services for Other Medical Devices
- 7.4.2. Analysis by Year of Establishment
- 7.4.3. Analysis by Size of Employee Base
- 7.4.4. Analysis by Location of Headquarters
- 7.4.5. Analysis by Location of Manufacturing Facility
- 7.4.6. Analysis by Regulatory Certifications / Accreditations
- 7.4.7. Analysis by Production Services Offered
- 7.4.8. Analysis by Post-Production Services Offered
- 7.4.9. Analysis by Other Services Offered
- 7.4.10. Analysis by Device Class
- 7.4.11. Analysis by Type of Material(s) Handled
- 7.4.12. Analysis by Scale of Operation
8. BENCHMARK ANALYSIS
- 8.1. Chapter Overview
- 8.2. Benchmark Analysis: Methodology
- 8.3. Region-wise Benchmark Analysis
- 8.3.1. North America, Peer Group I
- 8.3.2. North America, Peer Group II
- 8.3.3. North America, Peer Group III
- 8.3.4. North America, Peer Group IV
- 8.3.5. North America, Peer Group V
- 8.3.6. North America, Peer Group VI
- 8.3.7. North America, Peer Group VII
- 8.3.8. Europe, Peer Group VIII
- 8.3.9. Europe, Peer Group IX
- 8.3.10. Europe, Peer Group X
- 8.3.11. Asia, Peer Group XI
- 8.3.12. Asia, Peer Group XII
- 8.4. Concluding Remarks
9. SUPPLY CHAIN ANALYSIS
- 9.1. Chapter Overview
- 9.2. Overview of the Medical Device Supply Chain
- 9.3. Factors Affecting the Medical Device Supply Chain
- 9.4. Key Performance Indicators
- 9.5. Supply Chain Improvement Strategies
- 9.5.1. Optimization of Supply Chain Capabilities
- 9.5.2. Visible Supply Chain and Related Advantages
- 9.5.2.1. Augmenting Supply Chain Visibility Through Digitalization
- 9.5.2.2. Benefits of Digital Supply Chain
- 9.6. Effects of Supply Chain Optimization
- 9.7. Cost Analysis
- 9.7.1. Production-related Costs
- 9.7.1.1. Labor Costs
- 9.7.1.2. Raw Materials Related Costs
- 9.7.1.3. Regulatory Compliance
- 9.7.1.4. Technology-related Costs
- 9.7.2. Supply Chain-related Costs
- 9.7.2.1. Local Presence
- 9.7.2.2. Geopolitical Risk
- 9.7.2.3. Logistics
- 9.7.1. Production-related Costs
10. COMPANY PROFILES
- 10.1. Chapter Overview
- 10.2. CMOs Headquartered in North America
- 10.2.1. Cirtec Medical
- 10.2.1.1. Company Overview
- 10.2.1.2. Service Portfolio
- 10.2.1.3. Manufacturing Capabilities and Facilities
- 10.2.1.3.1. Facilities located in North America
- 10.2.1.3.2. Facilities Located in Europe
- 10.2.1.4. Future Outlook
- 10.2.2. DynaFlex Technologies
- 10.2.2.1. Company Overview
- 10.2.2.2. Service Portfolio
- 10.2.2.3. Manufacturing Capabilities and Facilities
- 10.2.2.4. Future Outlook
- 10.2.3. I-Tek Medical Technologies
- 10.2.3.1. Company Overview
- 10.2.3.2. Service Portfolio
- 10.2.3.3. Manufacturing Capabilities and Facilities
- 10.2.3.4. Future Outlook
- 10.2.4. Keystone Solutions Group
- 10.2.4.1. Company Overview
- 10.2.4.2. Service portfolio
- 10.2.4.3. Manufacturing Facilities
- 10.2.4.4. Future Outlook
- 10.2.5. Oscor
- 10.2.5.1. Company Overview
- 10.2.5.2. Service Portfolio
- 10.2.5.3. Manufacturing Capabilities and facilities
- 10.2.5.4. Future Outlook
- 10.2.6. SMC
- 10.2.6.1. Company Overview
- 10.2.6.2. Service Portfolio
- 10.2.6.3. Manufacturing Capabilities and facilities
- 10.2.6.3.1. Facilities Located in North America
- 10.2.6.3.2. Facility Located in Central America
- 10.2.6.3.3. Facility Located in Europe
- 10.2.6.3.4. Facility Located in Asia
- 10.2.6.4. Future Outlook
- 10.2.7. Stellartech Research
- 10.2.7.1. Company Overview
- 10.2.7.2. Service Portfolio
- 10.2.7.3. Manufacturing Capabilities and facilities
- 10.2.7.4. Future Outlook
- 10.2.1. Cirtec Medical
- 10.3. CMOs Headquartered in Europe
- 10.3.1. Creganna Medical
- 10.3.1.1. Company Overview
- 10.3.1.2. Service Portfolio
- 10.3.1.3. Manufacturing Facilities
- 10.3.1.3.1. Facilities Located in North America
- 10.3.1.3.2. Facilities Located in Central America
- 10.3.1.3.3. Facilities Located in Europe
- 10.3.1.3.4. Facilities Located in Asia
- 10.3.1.4. Future Outlook
- 10.3.2. Europlaz Technologies
- 10.3.2.1. Company Overview
- 10.3.2.2. Service Portfolio
- 10.3.2.3. Manufacturing facilities
- 10.3.2.4. Future Outlook
- 10.3.3. Riverside Medical Packaging
- 10.3.3.1. Company Overview
- 10.3.3.2. Service Portfolio
- 10.3.3.3. Manufacturing Facilities
- 10.3.3.4. Future Outlook
- 10.3.4. Synecco
- 10.3.4.1. Company Overview
- 10.3.4.2. Service Portfolio
- 10.3.4.3. Manufacturing Facilities
- 10.3.4.3.1. Facilities Located in Europe
- 10.3.4.3.2. Facilities Located in Asia
- 10.3.4.4. Future Outlook
- 10.3.5. Trelleborg Sealing Solutions
- 10.3.5.1. Company Overview
- 10.3.5.2. Service Portfolio
- 10.3.5.3. Manufacturing Facilities
- 10.3.5.3.1. Facilities Located in North America
- 10.3.5.3.2. Facilities Located in Europe
- 10.3.5.4. Future Outlook
- 10.3.1. Creganna Medical
- 10.4. CMOs Headquartered in Asia
- 10.4.1. Interplex
- 10.4.1.1. Company Overview
- 10.4.1.2. Service Portfolio
- 10.4.1.3. Manufacturing Facilities
- 10.4.1.3.1. Facilities Located in North America
- 10.4.1.3.2. Facilities Located in Europe
- 10.4.1.3.3. Facilities Located in Asia
- 10.4.1.4. Future Outlook
- 10.4.2. Modern Medical
- 10.4.2.1. Company Overview
- 10.4.2.2. Service Portfolio
- 10.4.2.3. Manufacturing facilities
- 10.4.2.4. Future Outlook
- 10.4.3. Providence Enterprise
- 10.4.3.1. Company Overview
- 10.4.3.2. Service Portfolio
- 10.4.3.3. Manufacturing Facilities
- 10.4.3.4. Future Outlook
- 10.4.4. Suzhou JenitekJenitek
- 10.4.4.1. Company overview
- 10.4.4.2. Service Portfolio
- 10.4.4.3. Manufacturing Facilities
- 10.4.4.4. Future Outlook
- 10.4.1. Interplex
11. CLINICAL TRIAL ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Clinical Trial Analysis: Word Cloud of Trial Titles
- 11.4. Analysis of Clinical Trials
- 11.4.1. Analysis by Trial Start Year
- 11.4.2. Analysis by Trial Status
- 11.4.3. Analysis by Phase of Development
- 11.4.4. Analysis by Target Indication
- 11.4.5. Analysis by Type of Sponsor
- 11.4.6. Analysis by Geography
- 11.4.7. Analysis by Trial Start Year and Geography
- 11.5. Analysis of Enrolled Patient Population
- 11.5.1. Analysis by Trial Status
- 11.5.2. Analysis by Phase of Development
- 11.5.3. Analysis by Geography
- 11.6. Analysis of Focus Therapeutic Areas
- 11.6.1. Analysis by Type of Sponsor
- 11.6.2. Analysis by Geography
- 11.6.2.1. North American Scenario
- 11.6.2.2. European Scenario
- 11.6.2.3. Asia Pacific Scenario
- 11.6.2.4. Rest of the World Scenario
- 11.6.3. Cardiovascular Disorders
- 11.6.3.1. Analysis by Trial Start Year
- 11.6.3.2. Analysis by Type of Sponsor
- 11.6.3.3. Analysis by Geography
- 11.6.4. Oncological Disorders
- 11.6.4.1. Analysis by Trial Start Year
- 11.6.4.2. Analysis by Type of Sponsor
- 11.6.4.3. Analysis by Geography
- 11.6.5. Central Nervous System (CNS) Disorders
- 11.6.5.1. Analysis by Trial Start Year
- 11.6.5.2. Analysis by Type of Sponsor
- 11.6.5.3. Analysis by Geography
- 11.6.6. Orthopedic Disorders
- 11.6.6.1. Analysis by Trial Start Year
- 11.6.6.2. Analysis by Type of Sponsor
- 11.6.6.3. Analysis by Geography
- 11.6.7. Respiratory Disorders
- 11.6.7.1. Analysis by Trial Start Year
- 11.6.7.2. Analysis by Type of Sponsor
- 11.6.7.3. Analysis by Geography
12. MERGERS AND ACQUISITIONS
- 12.1. Chapter Overview
- 12.2. Merger and Acquisition Models
- 12.3. Medical Device Contract Manufacturing: Mergers and Acquisitions
- 12.3.1. Analysis by Year of Mergers and Acquisitions
- 12.3.2. Analysis by Type of Acquisition
- 12.3.3. Most Active Acquirers: Analysis by Number of Acquisitions
- 12.3.4. Analysis by Key Value Drivers
- 12.3.4.1. Continent-wise distribution
- 12.3.4.2. Intercontinental and Intracontinental Deals
- 12.3.4.3. Country-wise distribution
- 12.3.5. Ownership Change Matrix
- 12.3.6. Acquisitions Made by Medical Device Companies
- 12.3.6.1. Acquisition of Entire Company
- 12.3.6.1.1. Analysis by Key Value Drivers
- 12.3.6.1.2. Analysis by Key Value Drivers and Year of Acquisition
- 12.3.6.1.3. Analysis by Type of Services Offered
- 12.3.6.1.4. Analysis by Type of Device
- 12.3.6.1.5. Analysis by Therapeutic Area
- 12.3.6.2. Acquisition of Company Assets
- 12.3.6.2.1. Analysis by Key Value Drivers
- 12.3.6.2.2. Analysis by Key Value Drivers and Year of Acquisition
- 12.3.6.1. Acquisition of Entire Company
- 12.3.7. Acquisition by Private Equity Firms
- 12.3.7.1. Analysis by Year of Acquisition
- 12.3.7.2. Analysis by Type of Acquisition
- 12.3.7.3. Analysis by Type of Device
- 12.3.7.4. Analysis by Therapeutic Area
- 12.4. Key Acquisitions: Deal Multiples
- 12.4.1. Analysis by Revenues
13. CASE STUDY: OFFSHORING MEDICAL DEVICE CONTRACT MANUFACTURING
- 13.1. Chapter Overview
- 13.2. Emerging Markets: Definitions and Key Regions
- 13.3. Role of Emerging Markets in Industrial Manufacturing
- 13.3.1. Contributions to the Automobile Industry
- 13.3.2. Contributions to the Aerospace and Defense Industries
- 13.4. Medical Devices Manufacturing in Emerging Markets
- 13.4.1. The US-Chinese Scenario
- 13.4.2. Partnership with Companies in Emerging Markets
- 13.5. Multinational Companies Focused on Manufacturing Medical Devices in Emerging Markets
- 13.6. Business Models for Medical Device Manufacturing in Emerging Markets
- 13.6.1. Case Studies
- 13.6.1.1. Study A: Overview
- 13.6.1.1.1. Value Proposition
- 13.6.1.1.2. Value Creation
- 13.6.1.2. Study B: Overview
- 13.6.1.2.1. Value Proposition
- 13.6.1.2.2. Value Creation
- 13.6.1.1. Study A: Overview
- 13.6.1. Case Studies
- 13.7. Key Challenges related to Innovation in the Medical Device Industry
- 13.7.1. Limitations of In-House Innovation
- 13.8. Advantages of Establishing Innovation Hubs in Emerging Markets
- 13.8.1. Challenges and Market Restraints
- 13.8.2. Strategies to Address Existing Challenges
- 13.9. Future Perspectives
14. MARKET FORECAST
- 14.1. Chapter Overview
- 14.2. Forecast Methodology and Key Assumptions
- 14.3. Overall Medical Device Contract Manufacturing Market, till 2035
- 14.3.1. Medical Device Contract Manufacturing Market: Distribution by Application Areas, till 2035
- 14.3.1.1. Medical Device Contract Manufacturing Market for Therapeutic Devices, till 2035
- 14.3.1.2. Medical Device Contract Manufacturing Market for Diagnostic Devices, till 2035
- 14.3.1.3. Medical Device Contract Manufacturing Market for Drug Delivery Devices, till 2035
- 14.3.1.4. Medical Device Contract Manufacturing Market for Other Devices, till 2035
- 14.3.2. Medical Device Contract Manufacturing Market: Distribution by Device Class, till 2035
- 14.3.2.1. Medical Device Contract Manufacturing Market: Class I Devices, till 2035
- 14.3.2.2. Medical Device Contract Manufacturing Market: Class II Devices, till 2035
- 14.3.2.3. Medical Device Contract Manufacturing Market: Class III Devices, till 2035
- 14.3.3. Medical Device Contract Manufacturing Market: Leading Players, till 2035
- 14.3.4. Medical Device Contract Manufacturing Market: Distribution by Therapeutic Areas, till 2035
- 14.3.4.1. Medical Device Contract Manufacturing Market for Cardiovascular Disorders, till 2035
- 14.3.4.2. Medical Device Contract Manufacturing Market for CNS Disorders, till 2035
- 14.3.4.3. Medical Device Contract Manufacturing Market for Metabolic Disorders, till 2035
- 14.3.4.4. Medical Device Contract Manufacturing Market for Oncological Disorders, till 2035
- 14.3.4.5. Medical Device Contract Manufacturing Market for Orthopedic Disorders, till 2035
- 14.3.4.6. Medical Device Contract Manufacturing Market for Ophthalmic Disorders, till 2035
- 14.3.4.7. Medical Device Contract Manufacturing Market for Pain Disorders, till 2035
- 14.3.4.8. Medical Device Contract Manufacturing Market for Respiratory Disorders, till 2035
- 14.3.4.9. Medical Device Contract Manufacturing Market for Other Therapeutic Areas, till 2035
- 14.3.1. Medical Device Contract Manufacturing Market: Distribution by Application Areas, till 2035
- 14.4. Medical Device Contract Manufacturing Market: Distribution by Geography, till 2035
- 14.4.1. Medical Device Contract Manufacturing Market in North America, till 2035
- 14.4.1.1. Medical Device Contract Manufacturing Market in the US, till 2035
- 14.4.1.2. Medical Device Contract Manufacturing Market in Rest of North America, till 2035
- 14.4.1.3. Medical Device Contract Manufacturing Market in North America: Class I Devices, till 2035
- 14.4.1.4. Medical Device Contract Manufacturing Market in North America: Class II Devices, till 2035
- 14.4.1.5. Medical Device Contract Manufacturing Market in North America: Class III Devices, till 2035
- 14.4.1.6. Medical Device Contract Manufacturing Market in North America for Cardiovascular Disorders, till 2035
- 14.4.1.7. Medical Device Contract Manufacturing Market in North America for CNS Disorders, till 2035
- 14.4.1.8. Medical Device Contract Manufacturing Market in North America for Metabolic Disorders, till 2035
- 14.4.1.9. Medical Device Contract Manufacturing Market in North America for Oncological Disorders, till 2035
- 14.4.1.10. Medical Device Contract Manufacturing Market in North America for Orthopedic Disorders, till 2035
- 14.4.1.11. Medical Device Contract Manufacturing Market in North America for Ophthalmic Disorders, till 2035
- 14.4.1.12. Medical Device Contract Manufacturing Market in North America for Pain Disorders, till 2035
- 14.4.1.13. Medical Device Contract Manufacturing Market in North America for Respiratory Disorders, till 2035
- 14.4.1.14. Medical Device Contract Manufacturing Market for Other Therapeutic Areas in North America, till 2035
- 14.4.2. Medical Device Contract Manufacturing Market in Europe, till 2035
- 14.4.2.1. Medical Device Contract Manufacturing Market in Italy, till 2035
- 14.4.2.2. Medical Device Contract Manufacturing Market in Germany, till 2035
- 14.4.2.3. Medical Device Contract Manufacturing Market in France, till 2035
- 14.4.2.4. Medical Device Contract Manufacturing Market in Spain, till 2035
- 14.4.2.5. Medical Device Contract Manufacturing Market in the UK, till 2035
- 14.4.2.6. Medical Device Contract Manufacturing Market in Rest of Europe, till 2035
- 14.4.2.7. Medical Device Contract Manufacturing Market in Europe: Class I Devices, till 2035
- 14.4.2.8. Medical Device Contract Manufacturing Market in Europe: Class II Devices, till 2035
- 14.4.2.9. Medical Device Contract Manufacturing Market in Europe: Class III Devices, till 2035
- 14.4.2.10. Medical Device Contract Manufacturing Market in Europe for Cardiovascular Disorders, till 2035
- 14.4.2.11. Medical Device Contract Manufacturing Market in Europe for CNS Disorders, till 2035
- 14.4.2.12. Medical Device Contract Manufacturing Market in Europe for Metabolic Disorders, till 2035
- 14.4.2.13. Medical Device Contract Manufacturing Market in Europe for Oncological Disorders, till 2035
- 14.4.2.14. Medical Device Contract Manufacturing Market in Europe for Orthopedic Disorders, till 2035
- 14.4.2.15. Medical Device Contract Manufacturing Market in Europe for Ophthalmic Disorders, till 2035
- 14.4.2.16. Medical Device Contract Manufacturing Market in Europe for Pain Disorders, till 2035
- 14.4.2.17. Medical Device Contract Manufacturing Market in Europe for Respiratory Disorders, till 2035
- 14.4.2.18. Medical Device Contract Manufacturing Market in Europe for Other Therapeutic Areas, till 2035
- 14.4.3. Medical Device Contract Manufacturing Market in Asia Pacific, till 2035
- 14.4.3.1. Medical Device Contract Manufacturing Market in China, till 2035
- 14.4.3.2. Medical Device Contract Manufacturing Market in Japan, till 2035
- 14.4.3.3. Medical Device Contract Manufacturing Market in India, till 2035
- 14.4.3.4. Medical Device Contract Manufacturing Market in Rest of Asia Pacific, till 2035
- 14.4.3.5. Medical Device Contract Manufacturing Market in Asia Pacific: Class I Devices, till 2035
- 14.4.3.6. Medical Device Contract Manufacturing Market in Asia Pacific: Class II Devices, till 2035
- 14.4.3.7. Medical Device Contract Manufacturing Market in Asia Pacific: Class III Devices, till 2035
- 14.4.3.8. Medical Device Contract Manufacturing Market in Asia Pacific for Cardiovascular Disorders, till 2035
- 14.4.3.9. Medical Device Contract Manufacturing Market in Asia Pacific for CNS Disorders, till 2035
- 14.4.3.10. Medical Device Contract Manufacturing Market in Asia Pacific for Metabolic Disorders, till 2035
- 14.4.3.11. Medical Device Contract Manufacturing Market in Asia Pacific for Oncological Disorders, till 2035
- 14.4.3.12. Medical Device Contract Manufacturing Market in Asia Pacific for Orthopedic Disorders, till 2035
- 14.4.3.13. Medical Device Contract Manufacturing Market in Asia Pacific for Ophthalmic Disorders, till 2035
- 14.4.3.14. Medical Device Contract Manufacturing Market in Asia Pacific for Pain Disorders, till 2035
- 14.4.3.15. Medical Device Contract Manufacturing Market in Asia Pacific for Respiratory Disorders, till 2035
- 14.4.3.16. Medical Device Contract Manufacturing Market in Asia Pacific for Other Therapeutic Areas, till 2035
- 14.4.4. Medical Device Contract Manufacturing Market in Rest of the World, till 2035
- 14.4.4.1. Medical Device Contract Manufacturing Market in Rest of the World: Class I Devices, till 2035
- 14.4.4.2. Medical Device Contract Manufacturing Market in Rest of the World: Class II Devices, till 2035
- 14.4.4.3. Medical Device Contract Manufacturing Market in Rest of the World: Class III Devices, till 2035
- 14.4.4.4. Medical Device Contract Manufacturing Market in Rest of the World for Cardiovascular Disorders, till 2035
- 14.4.4.5. Medical Device Contract Manufacturing Market in Rest of the World for CNS Disorders, till 2035
- 14.4.4.6. Medical Device Contract Manufacturing Market in Rest of the World for Metabolic Disorders, till 2035
- 14.4.4.7. Medical Device Contract Manufacturing Market in Rest of the World for Oncological Disorders, till 2035
- 14.4.4.8. Medical Device Contract Manufacturing Market in Rest of the World for Orthopedic Disorders, till 2035
- 14.4.4.9. Medical Device Contract Manufacturing Market in Rest of the World for Ophthalmic Disorders, till 2035
- 14.4.4.10. Medical Device Contract Manufacturing Market in Rest of the World for Pain Disorders, till 2035
- 14.4.4.11. Medical Device Contract Manufacturing Market in Rest of the World for Respiratory Disorders, till 2035
- 14.4.4.12. Medical Device Contract Manufacturing Market in Rest of the World for Other Therapeutic Areas, till 2035
- 14.4.1. Medical Device Contract Manufacturing Market in North America, till 2035
15. SWOT ANALYSIS
- 15.1. Chapter Overview
- 15.2. Strengths
- 15.3. Weaknesses
- 15.4. Opportunities
- 15.5. Threats
- 15.6. Concluding Remarks
16. CONCLUSION
- 16.1. Chapter Overview
- 16.2. Key Takeaways
17. SURVEY / INTERVIEW TRANSCRIPTS
18. APPENDIX 1: TABULATED DATA
19. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 4.1 Device Classification: US
- Table 4.2 Device Classification: Canada
- Table 4.3 HTA Processes in Canada: Provincial Requirements
- Table 4.4 Regulatory Bodies in EU5 Countries
- Table 4.5 Device Classification: Europe
- Table 4.6 List of CE Directives in Europe
- Table 4.7 Conformity Assessment Modules in Europe
- Table 4.8 Device Classification: Japan
- Table 4.9 Device Classification: China
- Table 4.10 Device Classification: Australia
- Table 4.11 Medical Devices: Regulatory Landscape Summary
- Table 5.1 List of Medical Device CMOs Offering Services for Therapeutic Devices
- Table 5.2 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Regulatory Certifications / Accreditations
- Table 5.3 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Manufacturing Services
- Table 5.4 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Post-Production Services
- Table 5.5 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Other Services
- Table 5.6 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Device Class
- Table 5.7 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Type of Material(s) Handled
- Table 5.8 Medical Device CMOs Offering Services for Therapeutic Devices: Information on Scale of Operation
- Table 6.1 List of Medical Device CMOs Offering Services for Diagnostic Devices
- Table 6.2 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Regulatory Certifications / Accreditations
- Table 6.3 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Manufacturing Services
- Table 6.4 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Post-Production Services
- Table 6.5 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Other Services
- Table 6.6 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Device Class
- Table 6.7 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Type of Material(s) Handled
- Table 6.8 Medical Device CMOs Offering Services for Diagnostic Devices: Information on Scale of Operations
- Table 7.1 List of Medical Device CMOs Offering Services for Drug Delivery Devices
- Table 7.2 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Regulatory Certifications / Accreditations
- Table 7.3 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Manufacturing Services
- Table 7.4 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Post-Production Services
- Table 7.5 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Other Services
- Table 7.6 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Device Class
- Table 7.7 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Type of Material(s) Handled
- Table 7.8 Medical Device CMOs Offering Services for Drug Delivery Devices: Information on Scale of Operations
- Table 7.9 List of Medical Device CMOs Offering Services for Other Devices
- Table 7.10 Medical Device CMOs Offering Services for Other Devices: Information on Regulatory Certifications / Accreditations
- Table 7.11 Medical Device CMOs Offering Services for Other Devices: Information on Manufacturing Services
- Table 7.12 Medical Device CMOs Offering Services for Other Devices: Information on Post-Production Services
- Table 7.13 Medical Device CMOs Offering Services for Other Devices: Information on Other Services
- Table 7.14 Medical Device CMOs Offering Services for Other Devices: Information on Device Class
- Table 7.15 Medical Device CMOs Offering Services for Other Devices: Information on Type of Material(s) Handled
- Table 7.16 Medical Device CMOs Offering Services for Other Devices: Information on Scale of Operations
- Table 8.1 Benchmark Analysis: Peer Groups
- Table 10.1 Cirtec Medical: Company Overview
- Table 10.2 DynaFlex Technologies: Company Overview
- Table 10.3 I-Tek Medical Technologies: Company Overview
- Table 10.4 Keystone Solutions Group: Company Overview
- Table 10.5 Oscor: Company Overview
- Table 10.6 SMC: Company Overview
- Table 10.7 Stellartech: Company Overview
- Table 10.8 Creganna Medical: Company Overview
- Table 10.9 Europlaz Technologies: Company Overview
- Table 10.10 Riverside Medical Packaging: Company Overview
- Table 10.11 Synecco: Post-production Company Overview
- Table 10.12 Trelleborg Sealing Solutions: Company Overview
- Table 10.13 Interplex: Company Overview
- Table 10.14 Modern Medical: Company Overview
- Table 10.15 Providence Enterprise: Company Overview
- Table 10.16 Suzhou Jenitek: Company Overview
- Table 12.1 Medical Device Contract Manufacturing: List of Mergers and Acquisitions, Since 2014
- Table 12.2 Mergers and Acquisitions: Key Value Drivers and Type of Devices, Since 2014
- Table 12.3 Mergers and Acquisitions: Deal Multiples, Since 2013
- Table 13.1 Comparative Analysis between Developed and Developing Nations
- Table 13.2 Medtech A: Company Overview
- Table 13.3 Medtech A: Operational Values
- Table 13.4 Medtech B: Company Overview
- Table 13.5 Medtech A: Operational Values
- Table 18.1 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Year of Establishment
- Table 18.2 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Size of Employee Base
- Table 18.3 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Location of Headquarters
- Table 18.4 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Location of Manufacturing Facility
- Table 18.5 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Regulatory Certifications / Accreditations
- Table 18.6 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Manufacturing Services Offered
- Table 18.7 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Post-Production Services Offered
- Table 18.8 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Other Services Offered
- Table 18.9 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Device Class
- Table 18.10 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Type of Material(s) Handled
- Table 18.11 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Scale of Operation
- Table 18.12 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Year of Establishment
- Table 18.13 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Size of Employee Base
- Table 18.14 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Location of Headquarters
- Table 18.15 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Location of Manufacturing Facility
- Table 18.16 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Regulatory Certifications / Accreditations
- Table 18.17 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Manufacturing Services Offered
- Table 18.18 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Post-Production Services Offered
- Table 18.19 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Other Services Offered
- Table 18.20 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Device Class
- Table 18.21 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Type of Material(s) Handled
- Table 18.22 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Scale of Operation
- Table 18.23 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Year of Establishment
- Table 18.24 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Size of Employee Base
- Table 18.25 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Location of Headquarters
- Table 18.26 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Location of Manufacturing Facility
- Table 18.27 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Regulatory Certifications / Accreditations
- Table 18.28 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Manufacturing Services Offered
- Table 18.29 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Post-Production Services Offered
- Table 18.30 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Other Services Offered
- Table 18.31 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Device Class
- Table 18.32 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Type of Material(s) Handled
- Table 18.33 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Scale of Operation
- Table 18.34 Medical Device CMOs Offering Services for Other Devices: Distribution by Year of Establishment
- Table 18.35 Medical Device CMOs Offering Services for Other Devices: Distribution by Size of Employee Base
- Table 18.36 Medical Device CMOs Offering Services for Other Devices: Distribution by Location of Headquarters
- Table 18.37 Medical Device CMOs Offering Services for Other Devices: Distribution by Location of Manufacturing Facility
- Table 18.38 Medical Device CMOs Offering Services for Other Devices: Distribution by Regulatory Certifications / Accreditations
- Table 18.39 Medical Device CMOs Offering Services for Other Devices: Distribution by Manufacturing Services Offered
- Table 18.40 Medical Device CMOs Offering Services for Other Devices: Distribution by Post-Production Services Offered
- Table 18.41 Medical Device CMOs Offering Services for Other Devices: Distribution by Other Services Offered
- Table 18.42 Medical Device CMOs Offering Services for Other Devices: Distribution by Device Class
- Table 18.43 Medical Device CMOs Offering Services for Other Devices: Distribution by Type of Material(s) Handled
- Table 18.44 Medical Device CMOs Offering Services for Other Devices: Distribution by Scale of Operation
- Table 18.45 Benchmark Analysis: Distribution by Region and Size of Employee Base
- Table 18.46 Clinical Trial Analysis: Distribution by Trial Start Year, Since 2018
- Table 18.47 Clinical Trial Analysis: Distribution by Trial Status
- Table 18.48 Clinical Trial Analysis: Distribution by Phase of Development
- Table 18.49 Clinical Trial Analysis: Top 10 Target Indication
- Table 18.50 Clinical Trial Analysis: Distribution by Sponsor Type
- Table 18.51 Clinical Trial Analysis: Distribution by Trial Start Year and Geography, Since 2018
- Table 18.52 Enrolled Patient Population: Distribution by Trial Status
- Table 18.53 Enrolled Patient Population: Distribution by Phase of Development
- Table 18.54 Clinical Trial Analysis: Distribution by Focus Therapeutic Areas
- Table 18.55 Heat Map Analysis: Emerging Therapeutic Areas
- Table 18.56 Clinical Trial Analysis: Distribution by Type of Sponsor and Top 10 Therapeutic Areas
- Table 18.57 Clinical Trial Analysis: Prominent Therapeutic Areas in North America
- Table 18.58 Clinical Trial Analysis: Prominent Therapeutic Areas in Europe
- Table 18.59 Clinical Trial Analysis: Prominent Therapeutic Areas in Asia Pacific
- Table 18.60 Clinical Trial Analysis: Prominent Therapeutic Areas in Rest of the World
- Table 18.61 Clinical Trials for Cardiovascular Disorders: Distribution by Trial Start Year, Since 2018
- Table 18.62 Clinical Trials for Cardiovascular Disorders: Distribution by Sponsor Type
- Table 18.63 Clinical Trials for Cardiovascular Disorders: Distribution by Geography
- Table 18.64 Clinical Trials for Oncological Disorders: Distribution of Trials by Start Year, Since 2018
- Table 18.65 Clinical Trials for Oncological Disorders: Distribution by Sponsor Type
- Table 18.66 Clinical Trials for Oncological Disorders: Distribution by Geography
- Table 18.67 Clinical Trials for CNS Disorders: Distribution of Trials by Start Year, Since 2018
- Table 18.68 Clinical Trials for CNS Disorders: Distribution by Sponsor Type
- Table 18.69 Clinical Trials for CNS Disorders: Distribution by Geography
- Table 18.70 Clinical Trials for Orthopedic Disorders: Distribution of Trials by Start Year, Since 2018
- Table 18.71 Clinical Trials for Orthopedic Disorders: Distribution by Sponsor Type
- Table 18.72 Clinical Trials for Orthopedic Disorders: Distribution by Geography
- Table 18.73 Clinical Trials for Respiratory Disorders: Distribution of Trials by Start Year, Since 2018
- Table 18.74 Clinical Trials for Respiratory Disorders: Distribution by Sponsor Type
- Table 18.75 Clinical Trials for Respiratory Disorders: Distribution by Geography
- Table 18.76 Mergers and Acquisitions: Year-Wise Cumulative Trend, Since 2018
- Table 18.77 Mergers and Acquisitions: Distribution by Type of Acquisitions
- Table 18.78 Acquisitions: Most Active Acquirers
- Table 18.79 Mergers and Acquisitions: Key Value Drivers
- Table 18.80 Mergers and Acquisitions: Distribution by Key Value Drivers and Year of Acquisition
- Table 18.81 Mergers and Acquisitions: Distribution by Type of Devices
- Table 18.82 Mergers and Acquisitions: Distribution by Therapeutic Area
- Table 18.83 Mergers and Acquisitions: Distribution by Services Offered
- Table 18.84 Acquisitions: Country-wise Distribution
- Table 18.85 Overall Medical Device CMOs Market, Base Scenario, till 2035 (USD Billion)
- Table 18.86 Medical Device CMOs Market for Therapeutic Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.87 Medical Device CMOs Market for Diagnostic Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.88 Medical Device CMOs Market for Drug Delivery Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.89 Medical Device CMOs Market for Other Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.90 Medical Device CMOs Market for Class I Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.91 Medical Device CMOs Market for Class II Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.92 Medical Device CMOs Market for Class III Devices, Base Scenario, till 2035 (USD Billion)
- Table 18.93 Medical Device CMOs Market: Leading Players, till 2035 (USD Billion)
- Table 18.94 Medical Device CMOs Market for Cardiovascular Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.95 Medical Device CMOs Market for CNS Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.96 Medical Device CMOs Market for Metabolic Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.97 Medical Device CMOs Market for Oncological Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.98 Medical Device CMOs Market for Orthopedic Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.99 Medical Device CMOs Market for Ophthalmic Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.100 Medical Device CMOs Market for Pain Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.101 Medical Device CMOs Market for Respiratory Disorders, Base Scenario, till 2035 (USD Billion)
- Table 18.102 Medical Device CMOs Market for Other Therapeutic Areas, Base Scenario, till 2035 (USD Billion)
- Table 18.103 Medical Device CMOs Market in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.104 Medical Device CMOs Market in the US, till 2035 (USD Billion)
- Table 18.105 Medical Device CMOs Market in Rest of North America, till 2035 (USD Billion)
- Table 18.106 Medical Device CMOs Market for Class I Devices in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.107 Medical Device CMOs Market for Class II Devices in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.108 Medical Device CMOs Market for Class III Devices in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.109 Medical Device CMOs Market for Cardiovascular Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.110 Medical Device CMOs Market for CNS Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.111 Medical Device CMOs Market for Metabolic Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.112 Medical Device CMOs Market for Oncological Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.113 Medical Device CMOs Market for Orthopedic Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.114 Medical Device CMOs Market for Ophthalmic Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.115 Medical Device CMOs Market for Pain Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.116 Medical Device CMOs Market for Respiratory Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.117 Medical Device CMOs Market for Other Therapeutic Areas in North America, Base Scenario, till 2035 (USD Billion)
- Table 18.118 Medical Device CMOs Market in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.119 Medical Device CMOs Market in Italy, Base Scenario, till 2035 (USD Billion)
- Table 18.120 Medical Device CMOs Market in Germany, Base Scenario, till 2035 (USD Billion)
- Table 18.121 Medical Device CMOs Market in France, Base Scenario, till 2035 (USD Billion)
- Table 18.122 Medical Device CMOs Market in Spain, Base Scenario, till 2035 (USD Billion)
- Table 18.123 Medical Device CMOs Market in the UK, Base Scenario, till 2035 (USD Billion)
- Table 18.124 Medical Device CMOs Market in Rest of Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.125 Medical Device CMOs Market for Class I Devices in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.126 Medical Device CMOs Market for Class II Devices in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.127 Medical Device CMOs Market for Class III Devices in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.128 Medical Device CMOs Market for Cardiovascular Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.129 Medical Device CMOs Market for CNS Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.130 Medical Device CMOs Market for Metabolic Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.131 Medical Device CMOs Market of Oncological Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.132 Medical Device CMOs Market for Orthopedic Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.133 Medical Device CMOs Market for Ophthalmic Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.134 Medical Device CMOs Market for Pain Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.135 Medical Device CMOs Market for Respiratory Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.136 Medical Device CMOs Market for Other Therapeutic Areas in Europe, Base Scenario, till 2035 (USD Billion)
- Table 18.137 Medical Device CMOs Market in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.138 Medical Device CMOs Market in China, Base Scenario, till 2035 (USD Billion)
- Table 18.139 Medical Device CMOs Market in Japan, Base Scenario, till 2035 (USD Billion)
- Table 18.140 Medical Device CMOs Market in India, Base Scenario, till 2035 (USD Billion)
- Table 18.141 Medical Device CMOs Market in Rest of Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.142 Medical Device CMOs Market for Class I Devices in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.143 Medical Device CMOs Market for Class II Devices in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.144 Medical Device CMOs Market for Class III Devices in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.145 Medical Device CMOs Market for Cardiovascular Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.146 Medical Device CMOs Market for CNS Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.147 Medical Device CMOs Market for Metabolic Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.148 Medical Device CMOs Market for Oncological Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.149 Medical Device CMOs Market for Orthopedic Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.150 Medical Device CMOs Market for Ophthalmic Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.151 Medical Device CMOs Market for Pain Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.152 Medical Device CMOs Market for Respiratory Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Table 18.153 Medical Device CMOs Market for Other Therapeutic Areas in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
List of Figures
- Figure 3.1 Historical Evolution of Medical Devices
- Figure 3.2 Medical Device Manufacturing Process
- Figure 3.3 Challenges Associated with Medical Device Manufacturing
- Figure 3.4 Advantages of Automation
- Figure 3.5 Services Offered by CMOs
- Figure 3.6 Advantages of Outsourcing to CMOs
- Figure 3.7 Risks Associated with Outsourcing to CMOs
- Figure 3.8 Key Considerations for Selecting a CMO Partner
- Figure 4.1 US FDA: Review / Approval Process
- Figure 4.2 Health Canada: Review / Approval Process
- Figure 4.3 Summary of CE Marking Process
- Figure 4.4 Pharmaceuticals and Medical Devices Agency: Review / Approval Process
- Figure 4.5 China Food and Drug Administration: Review / Approval Process
- Figure 4.6 Therapeutic Goods Administration: Review / Approval Process
- Figure 4.7 Bubble Analysis: Regional Regulatory Environment
- Figure 5.1 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Year of Establishment
- Figure 5.2 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Size of Employee Base
- Figure 5.3 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Location of Headquarters
- Figure 5.4 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Location of Manufacturing Facility
- Figure 5.5 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Regulatory Certifications / Accreditations
- Figure 5.6 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Manufacturing Services Offered
- Figure 5.7 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Post-Production Services Offered
- Figure 5.8 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Other Services Offered
- Figure 5.9 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Device Class
- Figure 5.10 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Type of Material(s) Handled
- Figure 5.11 Medical Device CMOs Offering Services for Therapeutic Devices: Distribution by Scale of Operation
- Figure 6.1 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Year of Establishment
- Figure 6.2 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Size of Employee Base
- Figure 6.3 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Location of Headquarters
- Figure 6.4 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Location of Manufacturing Facility
- Figure 6.5 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Regulatory Certifications / Accreditations
- Figure 6.6 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Manufacturing Services Offered
- Figure 6.7 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Post-Production Services Offered
- Figure 6.8 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Other Services Offered
- Figure 6.9 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Device Class
- Figure 6.10 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Type of Material(s) Handled
- Figure 6.11 Medical Device CMOs Offering Services for Diagnostic Devices: Distribution by Scale of Operation
- Figure 7.1 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Year of Establishment
- Figure 7.2 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Size of Employee Base
- Figure 7.3 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Location of Headquarters
- Figure 7.4 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Location of Manufacturing Facility
- Figure 7.5 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Regulatory Certifications / Accreditations
- Figure 7.6 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Manufacturing Services Offered
- Figure 7.7 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Post-Production Services Offered
- Figure 7.8 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Other Services Offered
- Figure 7.9 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Device Class
- Figure 7.10 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Type of Material(s) Handled
- Figure 7.11 Medical Device CMOs Offering Services for Drug Delivery Devices: Distribution by Scale of Operation
- Figure 7.12 Medical Device CMOs Offering Services for Other Devices: Distribution by Year of Establishment
- Figure 7.13 Medical Device CMOs Offering Services for Other Devices: Distribution by Size of Employee Base
- Figure 7.14 Medical Device CMOs Offering Services for Other Devices: Distribution by Location of Headquarters
- Figure 7.15 Medical Device CMOs Offering Services for Other Devices: Distribution by Location of Manufacturing Facility
- Figure 7.16 Medical Device CMOs Offering Services for Other Devices: Distribution by Regulatory Certifications / Accreditations
- Figure 7.17 Medical Device CMOs Offering Services for Other Devices: Distribution by Manufacturing Services Offered
- Figure 7.18 Medical Device CMOs Offering Services for Other Devices: Distribution by Post-Production Services Offered
- Figure 7.19 Medical Device CMOs Offering Services for Other Devices: Distribution by Other Services Offered
- Figure 7.20 Medical Device CMOs Offering Services for Other Devices: Distribution by Device Class
- Figure 7.21 Medical Device CMOs Offering Services for Other Devices: Distribution by Type of Material(s) Handled
- Figure 7.22 Medical Device CMOs Offering Services for Other Devices: Distribution by Scale of Operation
- Figure 8.1 Benchmark Analysis: Distribution by Region and Size of Employee Base
- Figure 8.2 Benchmark Analysis: North America, Peer Group I
- Figure 8.3 Benchmark Analysis: North America, Peer Group II
- Figure 8.4 Benchmark Analysis: North America, Peer Group III
- Figure 8.5 Benchmark Analysis: North America, Peer Group IV
- Figure 8.6 Benchmark Analysis: North America, Peer Group V
- Figure 8.7 Benchmark Analysis: North America, Peer Group VI
- Figure 8.8 Benchmark Analysis: North America, Peer Group VII
- Figure 8.9 Benchmark Analysis: Europe, Peer Group VIII
- Figure 8.10 Benchmark Analysis: Europe, Peer Group IX
- Figure 8.11 Benchmark Analysis: Europe, Peer Group X
- Figure 8.12 Benchmark Analysis: Asia, Peer Group XI
- Figure 11.10 Enrolled Patient Population: Distribution by Phase of Development
- Figure 11.11 Enrolled Patient Population: Distribution by Geography
- Figure 11.12 Clinical Trial Analysis: Distribution by Focus Therapeutic Areas
- Figure 11.13 Heat Map Analysis: Emerging Therapeutic Areas
- Figure 11.14 Clinical Trial Analysis: Distribution by Type of Sponsor and Top 10 Therapeutic Areas
- Figure 11.15 Clinical Trial Analysis: Prominent Therapeutic Areas in North America
- Figure 11.16 Clinical Trial Analysis: Prominent Therapeutic Areas in Europe
- Figure 11.17 Clinical Trial Analysis: Prominent Therapeutic Areas in Asia Pacific
- Figure 11.18 Clinical Trial Analysis: Prominent Therapeutic Areas in Rest of the World
- Figure 11.19 Clinical Trials for Cardiovascular Disorders: Distribution by Trial Start Year, Since 2018
- Figure 11.20 Clinical Trials for Cardiovascular Disorders: Distribution by Sponsor Type
- Figure 11.21 Clinical Trials for Cardiovascular Disorders: Distribution by Geography
- Figure 11.22 Clinical Trials for Oncological Disorders: Distribution of Trials by Start Year, Since 2018
- Figure 11.23 Clinical Trials for Oncological Disorders: Distribution by Sponsor Type
- Figure 11.24 Clinical Trials for Oncological Disorders: Distribution by Geography
- Figure 11.25 Clinical Trials for CNS Disorders: Distribution of Trials by Start Year, Since 2018
- Figure 11.26 Clinical Trials for CNS Disorders: Distribution by Sponsor Type
- Figure 11.27 Clinical Trials for CNS Disorders: Distribution by Geography
- Figure 11.28 Clinical Trials for Orthopedic Disorders: Distribution of Trials by Start Year, Since 2018
- Figure 11.29 Clinical Trials for Orthopedic Disorders: Distribution by Sponsor Type
- Figure 11.30 Clinical Trials for Orthopedic Disorders: Distribution by Geography
- Figure 11.31 Clinical Trials for Respiratory Disorders: Distribution of Trials by Start Year, Since 2018
- Figure 11.32 Clinical Trials for Respiratory Disorders: Distribution by Sponsor Type
- Figure 11.33 Clinical Trials for Respiratory Disorders: Distribution by Geography
- Figure 12.1 Mergers and Acquisitions: Year-Wise Cumulative Trend, Since 2018
- Figure 12.2 Mergers and Acquisitions: Distribution by Type of Acquisitions
- Figure 12.3 Most Active Acquirers: Distribution by Number of Acquisitions
- Figure 12.4 Mergers and Acquisitions: Key Value Drivers
- Figure 12.5 Mergers and Acquisitions: Distribution by Key Value Drivers and Year of Acquisition
- Figure 12.6 Mergers and Acquisitions: Distribution by Type of Device
- Figure 12.7 Heat Map Analysis: Type of Devices and Key Value Drivers
- Figure 12.8 Mergers and Acquisitions: Distribution by Therapeutic Area
- Figure 12.9 Mergers and Acquisitions: Distribution by Services Offered
- Figure 12.10 Acquisitions: Continental Distribution
- Figure 12.11 Acquisitions: Country-wise Distribution
- Figure 12.12 Acquisitions: Intercontinental and Intracontinental Acquisitions
- Figure 12.13 Mergers and Acquisitions: Relationship between Deal Multiples
- Figure 13.1 Prominent Emerging Markets
- Figure 13.2 Recommendations to Optimize Manufacturing Strategies
- Figure 14.1 Overall Medical Device CMOs Market, Base Scenario, till 2035 (USD Billion)
- Figure 14.2 Medical Device CMOs Market for Therapeutic Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.3 Medical Device CMOs Market for Diagnostic Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.4 Medical Device CMOs Market for Drug Delivery Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.5 Medical Device CMOs Market for Other Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.6 Medical Device CMOs Market for Class I Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.7 Medical Device CMOs Market for Class II Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.8 Medical Device CMOs Market for Class III Devices, Base Scenario, till 2035 (USD Billion)
- Figure 14.9 Medical Device CMOs Market: Leading Players, till 2035 (USD Billion)
- Figure 14.10 Medical Device CMOs Market for Cardiovascular Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.11 Medical Device CMOs Market for CNS Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.12 Medical Device CMOs Market for Metabolic Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.13 Medical Device CMOs Market for Oncological Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.14 Medical Device CMOs Market for Orthopedic Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.15 Medical Device CMOs Market for Ophthalmic Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.16 Medical Device CMOs Market for Pain Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.17 Medical Device CMOs Market for Respiratory Disorders, Base Scenario, till 2035 (USD Billion)
- Figure 14.18 Medical Device CMOs Market for Other Therapeutic Areas, Base Scenario, till 2035 (USD Billion)
- Figure 14.19 Medical Device CMOs Market in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.20 Medical Device CMOs Market in the US, till 2035 (USD Billion)
- Figure 14.21 Medical Device CMOs Market in Rest of North America, till 2035 (USD Billion)
- Figure 14.22 Medical Device CMOs Market for Class I Devices in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.23 Medical Device CMOs Market for Class II Devices in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.24 Medical Device CMOs Market for Class III Devices in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.25 Medical Device CMOs Market for Cardiovascular Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.26 Medical Device CMOs Market for CNS Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.27 Medical Device CMOs Market for Metabolic Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.28 Medical Device CMOs Market for Oncological Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.29 Medical Device CMOs Market for Orthopedic Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.30 Medical Device CMOs Market for Ophthalmic Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.31 Medical Device CMOs Market for Pain Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.32 Medical Device CMOs Market for Respiratory Disorders in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.33 Medical Device CMOs Market for Other Therapeutic Areas in North America, Base Scenario, till 2035 (USD Billion)
- Figure 14.34 Medical Device CMOs Market in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.35 Medical Device CMOs Market in Italy, Base Scenario, till 2035 (USD Billion)
- Figure 14.36 Medical Device CMOs Market in Germany, Base Scenario, till 2035 (USD Billion)
- Figure 14.37 Medical Device CMOs Market in France, Base Scenario, till 2035 (USD Billion)
- Figure 14.38 Medical Device CMOs Market in Spain, Base Scenario, till 2035 (USD Billion)
- Figure 14.39 Medical Device CMOs Market in the UK, Base Scenario, till 2035 (USD Billion)
- Figure 14.40 Medical Device CMOs Market in Rest of Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.41 Medical Device CMOs Market for Class I Devices in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.42 Medical Device CMOs Market for Class II Devices in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.43 Medical Device CMOs Market for Class III Devices in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.44 Medical Device CMOs Market for Cardiovascular Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.45 Medical Device CMOs Market for CNS Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.46 Medical Device CMOs Market for Metabolic Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.47 Medical Device CMOs Market of Oncological Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.48 Medical Device CMOs Market for Orthopedic Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.49 Medical Device CMOs Market for Ophthalmic Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.50 Medical Device CMOs Market for Pain Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.51 Medical Device CMOs Market for Respiratory Disorders in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.52 Medical Device CMOs Market for Other Therapeutic Areas in Europe, Base Scenario, till 2035 (USD Billion)
- Figure 14.53 Medical Device CMOs Market in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.54 Medical Device CMOs Market in China, Base Scenario, till 2035 (USD Billion)
- Figure 14.55 Medical Device CMOs Market in Japan, Base Scenario, till 2035 (USD Billion)
- Figure 14.56 Medical Device CMOs Market in India, Base Scenario, till 2035 (USD Billion)
- Figure 14.57 Medical Device CMOs Market in Rest of Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.58 Medical Device CMOs Market for Class I Devices in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.59 Medical Device CMOs Market for Class II Devices in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.60 Medical Device CMOs Market for Class III Devices in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.61 Medical Device CMOs Market for Cardiovascular Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.62 Medical Device CMOs Market for CNS Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.63 Medical Device CMOs Market for Metabolic Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.64 Medical Device CMOs Market for Oncological Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.65 Medical Device CMOs Market for Orthopedic Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.66 Medical Device CMOs Market for Ophthalmic Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.67 Medical Device CMOs Market for Pain Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.68 Medical Device CMOs Market for Respiratory Disorders in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 14.69 Medical Device CMOs Market for Other Therapeutic Areas in Asia and Rest of the World, Base Scenario, till 2035 (USD Billion)
- Figure 15.1 Medical Device Contract Manufacturing SWOT Analysis: Overview
- Figure 15.2 Comparison of SWOT Factors: Harvey Ball Analysis








