 |
市場調查報告書
商品編碼
1737059
臨床試驗軟體市場:各部署類型,提供類別,各軟體功能,各終端用戶,各地區Clinical Trial Software Market Distribution by Type of Deployment, Type of Delivery, Features of Software and Geographical Regions |
||||||
預計到 2035 年,全球臨床試驗軟體市場規模將從目前的 6.9 億美元增長至 36.8 億美元,預測期內的複合年增長率為 14%。
市場區隔根據以下參數對市場規模和機會進行分類:
各部署類型
- 開雲端
- 內部部署
提供類別
- 網站為基礎的
- 遠端監控
各軟體功能
- EDC
- eCOA/ePRO
- eConsent
各終端用戶
- 製藥/生物科技產業
- 學術·研究機關
- 其他的產業
各地區
- 北美
- 歐洲
- 亞太地區
臨床試驗軟體市場:成長和趨勢
臨床試驗是前瞻性的生物醫學研究,旨在評估人體內的醫療、外科和行為幹預措施,並探索診斷和預防/治療疾病的新方法。為了獲得新型治療幹預措施的上市監管批准,需要高度準確且複雜的臨床試驗數據來驗證藥物針對特定適應症的安全性和有效性。然而,這種傳統的臨床研究方法面臨著許多課題,包括高昂的資本投入、較低的患者採用率以及參與各種治療幹預措施開發的公司缺乏可靠的數據。這導致無法有效率地產生充足的臨床證據,為藥物開發公司以及獲得救命治療的患者帶來了巨大的資金損失。事實上,傳統的手動臨床試驗幾乎佔據了藥物開發過程中50%的時間。在此背景下,臨床試驗軟體因其能夠即時分析、數據管理和追蹤藥物不良反應而備受關注。
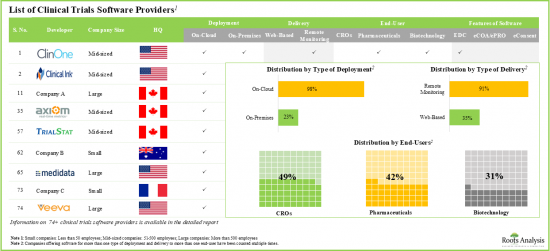
近年來,各公司開發了許多臨床試驗軟體/臨床試驗管理系統,包括EDC、eCOA/ePRO和eConsent。這些工具支援即時臨床試驗,並提高患者對藥物檢測和分析的依從性。由於其重要性,許多臨床試驗軟體市場參與者正在進入該領域並開發創新技術。值得注意的是,這些技術有助於提升臨床試驗結果,並減輕製藥公司的負擔。由於對自動化臨床試驗軟體的需求不斷增長,預計市場在預測期內將大幅增長。
臨床試驗軟體市場:關鍵洞察
本報告深入探討了臨床試驗軟體市場的現狀,並識別了行業內的潛在成長機會。主要發現包括:
- 全球超過 70 家公司聲稱已開發出能夠實現臨床研究方法分散化的軟體解決方案,從而優化臨床試驗的時間和成本。
- 目前的市場格局高度分散,現有企業和新進業者都提供具有先進功能的臨床試驗軟體。事實上,自 2000 年以來,已有超過 51 家專注於開發臨床試驗軟體的新創公司成立。
- 為了建立競爭優勢,各公司正積極擴展現有能力,增強各自的產品線,並緊跟不斷變化的產業基準。
- 由於預期獲得豐厚回報,許多公私投資者紛紛大舉投資,促使融資活動激增。尤其是北美,該領域融資案例約佔90%。
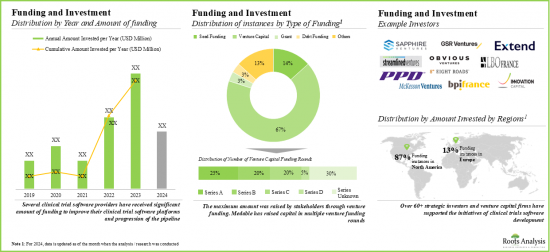
- 過去幾年,該領域的合作夥伴關係和協作顯著增加,顯示利害關係人的興趣日益濃厚。
- 值得注意的是,大多數併購案例發生在2023年,顯示該領域正逐漸轉向整合。此外,大多數交易(87%)是收購,其次是合併。
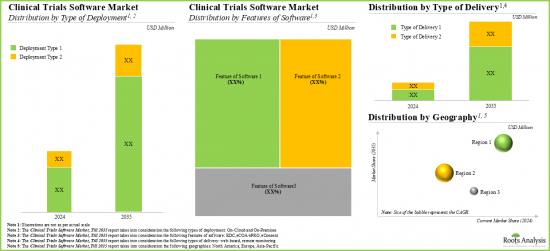
- 預計未來十年該市場將以每年14%的速度成長。機會可能因部署類型、軟體功能、產品類型和地理區域而異。
臨床試驗軟體市場:關鍵細分市場
電子同意書 (eConsent) 軟體細分市場佔據全球臨床試驗軟體市場的最大佔有率
依軟體功能劃分,全球臨床試驗軟體市場分為 EDC、eCOA/ePRO 和 eConsent 軟體。目前,eConsent 軟體佔據臨床試驗軟體市場的絕大部分佔有率。值得注意的是,eCOA/ePRO 細分市場的全球臨床試驗軟體市場很可能會以相對較高的複合年增長率成長。
依地區劃分,市場分為北美、歐洲和亞太地區。目前來看,北美佔據最大市場佔有率,而亞太地區市場預計在預測期內將呈現良好成長。
本報告提供全球臨床試驗軟體市場相關調查,提供市場概要,以及各部署類型,提供類別,各軟體功能,各終端用戶,各地區的趨勢,及加入此市場的主要企業簡介等資訊。
目錄
第1章 序文
第2章 摘要整理
第3章 簡介
- 章概要
- 臨床研究的現有的規定
- 虛擬臨床試驗
- 虛擬臨床試驗管理相關的機會與課題
- 未來展望
第4章 市場形勢:臨床試驗軟體市場
- 章概要
- 臨床試驗軟體市場:產品清單
- 臨床試驗軟體市場:開發商的形勢
第5章 北美的臨床試驗軟體開發公司:企業簡介
- 章概要
- Advarra
- Arisglobal
- AssistRx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
第6章 歐洲的臨床試驗軟體開發企業:企業簡介
- 章概要
- Calyx
第7章 企業競爭力分析
第8章 夥伴關係和合作
第9章 合併和收購
第10章 與資金籌措投資分析
第11章 與市場預測機會分析
- 章概要
- 預測調查手法主要的前提條件
- 全球臨床試驗軟體市場,2021年~2035年
- 臨床試驗軟體市場,2021年~2035年:各部署類型分佈
- 臨床試驗軟體市場,2021年~2035年:提供類別分佈
- 臨床試驗軟體市場,2021年~2035年:各軟體功能分佈
- 臨床試驗軟體市場,2021年~2035年:各地區分佈
第12章 結論
第13章 附錄1:表格形式的資料
第14章 附錄2:企業·團體一覽
CLINICAL TRIAL SOFTWARE MARKET: OVERVIEW
As per Roots Analysis, the global clinical trial software market is estimated to grow from USD 0.69 billion in the current year to USD 3.68 billion by 2035, at a CAGR of 14% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Deployment
- On-Cloud
- On-Premises
Type of Delivery
- Web-Based
- Remote Monitoring
Features of Software
- EDC
- eCOA/ePRO
- eConsent
End Users
- Pharmaceutical / Biotechnology Industries
- Academic and Research Institutes
- Other Industries
Geographical Regions
- North America
- Europe
- Asia-Pacific
CLINICAL TRIAL SOFTWARE MARKET: GROWTH AND TRENDS
Clinical trials are prospective biomedical research studies designed to evaluate medical, surgical or behavioral interventions in people and investigate novel approaches for the diagnosis and prevention / treatment of diseases. In order to gain marketing approval from regulatory authorities for a novel therapeutic intervention, highly accurate and elaborate clinical trial data is required to validate the drug's safety and effectiveness towards a specific target indication. However, there are several challenges associated with this traditional way of clinical research. Some of these include high capital investment, low patient recruitment rates and lack of robust data faced by companies involved in the development of various therapeutic interventions. This leads to inefficiency in generating sufficient clinical evidence, resulting in massive capital losses for drug developers, as well as patients accessing these life-saving therapies. In fact, manual conventional clinical trials consume almost 50% of the time during drug development. In this context, clinical trial software has gained significant attention owing to its ability for real-time analysis, data management, and tracking of the adverse impact of drugs.

In recent years, various players have developed a number of clinical trial software / clinical trial management systems, including EDC, eCOA / ePRO, and eConsent. These tools enable real-time clinical studies and improve patient compliance for drug testing and analysis. Owing to its significance, several clinical trial software market players are currently engaged in this field to develop innovative technologies. Notably, these technologies encourage successful outcomes of clinical trials and reduce the burden of pharmaceutical companies. Given the increasing demand for automated clinical trial software, the market is expected to witness substantial growth during the forecast period.
CLINICAL TRIAL SOFTWARE MARKET: KEY INSIGHTS
The report delves into the current state of the clinical trial software market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- 70+ companies worldwide claim to have developed software solutions allowing decentralization of the clinical research process, optimizing time and cost spent on clinical trials.
- The current market landscape is highly fragmented, with the presence of both established players and new entrants offering clinical trials software with advanced features. In fact, since 2000, over 51 start-ups focused on developing clinical trials software have been established.
- In pursuit of building a competitive edge, companies are actively expanding their existing capabilities to enhance their respective offerings and comply with evolving industry benchmarks.
- Foreseeing lucrative returns, many public and private investors have significant investments, marking a surge in funding activity. Notably, North America witnessed around 90% of funding instances in this domain.

- The domain has witnessed a considerable increase in partnerships and collaborations over the past few years, indicating the rising interest of stakeholders.
- Notably, most of the M&A instances were reported in 2023, indicating a gradual shift towards consolidation. Further, majority (87%) of agreements were acquisitions, followed by mergers.
- We expect the market to grow at an annualized rate of 14% in the coming decade; the opportunity is likely to be well distributed across type of deployment, features of software, type of delivery and geographical regions.

CLINICAL TRIAL SOFTWARE MARKET: KEY SEGMENTS
The eConsent Software Segment Holds the Largest Share of the Global Clinical Trial Software Market
Based on the features of software, the global market for clinical trial software is segmented into EDC, eCOA/ePRO and eConsent software. Currently, the majority of the clinical trial software market is captured by eConsent software. It is worth highlighting that the global clinical trial software market for eCOA / ePRO segment is likely to grow at a relatively higher CAGR.
North America Accounts for the Largest Share of the Market
Based on the geographical regions, the market is segmented into North America, Europe and Asia-Pacific. In the current scenario, North America is likely to capture the largest market share while the market in Asia-Pacific is anticipated to demonstrate lucrative growth during the forecast period.
Example Players in the Clinical Trial Software Market
- Advarra
- Arisglobal
- AssistRx
- Calyx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
CLINICAL TRIAL SOFTWARE MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global clinical trial software market, focusing on key market segments, including [A] type of deployment, [B] type of delivery, [C] features of software, [D] end users and [E] geographical regions.
- Clinical Trials Software Market Landscape: A comprehensive evaluation of clinical trials software market, based on several relevant parameters, such as [A] type of deployment, [B] type of delivery, [C] type of end-user, [D] features of software, [E] trial design and [F] type of technology. Additionally, a comprehensive evaluation of the companies engaged in developing clinical trials software, based on several relevant parameters, such as [G] year of establishment, [H] company and [I] location of headquarters.
- Company Profiles: In-depth profiles of key players engaged in the development of clinical trials software, focusing on [A] overview of the company, [B] product portfolio, and [C] recent developments and [D] an informed future outlook.
- Company Competitiveness Analysis: A comprehensive competitive analysis of clinical trials software developers, examining factors, such as [A] supplier strength, [B] product portfolio strength and [C] service applicability.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the global clinical trials software market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] geographical distribution of partnership activity and [D] most active players (in terms of the number of partnerships signed).
- Mergers and Acquisitions: An in-depth analysis of the mergers and acquisitions undertaken in this domain, based on relevant parameters, such as [A] type of agreement, [B] year of mergers and acquisitions, [C] geographical location and [D] most active players (in terms of the number of mergers and acquisitions).
- Funding and Investment Analysis: An in-depth analysis of the fundings raised by companies engaged in this domain, based on relevant parameters, such as [A] year of funding, [B] type of funding, [C] amount invested, [D] most active players and [F] most active investors.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Existing Constraints in Clinical Research
- 3.2.1. Increasing Trial Costs and Complexity
- 3.2.2. Evolving Regulatory Standards
- 3.2.3. Patient Recruitment and Retention-Related Challenges
- 3.2.4. Inefficient Data Handling
- 3.3. Virtual Clinical Trials
- 3.3.1. Electronic Data Capture Solutions
- 3.3.2. Electronic Clinical Outcome Assessment and Electronic Patient Reported Outcome Solutions (eCOA / ePRO)
- 3.3.3. Electronic Consent Solutions
- 3.4. Opportunities and challenges associated with Virtual Clinical Trials Management
- 3.5 Future Perspectives
4 MARKET LANDSCAPE: CLINICAL TRIALS SOFTWARE MARKET
- 4.1. Chapter Overview
- 4.2. Clinical Trials Software Market: List of Products
- 4.2.1. Analysis by Type of Deployment
- 4.2.2. Analysis by Type of Delivery
- 4.2.3. Analysis by End-User
- 4.2.4. Analysis by Features of Software
- 4.2.5. Analysis by Trial Design
- 4.2.6. Analysis by Type of Technology
- 4.3. Clinical Trials Software Market: Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Geography
5 CLINICAL TRIALS SOFTWARE DEVELOPERS IN NORTH AMERICA: COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Advarra
- 5.2.1. Company Overview
- 5.2.2. Product Portfolio: Clinical Trials Software
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Arisglobal
- 5.3.1. Company Overview
- 5.3.2. Product Portfolio: Clinical Trials Software
- 5.3.3. Recent Developments and Future Outlook
- 5.4. AssistRx
- 5.4.1. Company Overview
- 5.4.2. Product Portfolio: Clinical Trials Software
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Clario
- 5.5.1. Company Overview
- 5.5.2. Product Portfolio: Clinical Trials Software
- 5.5.3. Recent Developments and Future Outlook
- 5.6. IBM
- 5.6.1. Company Overview
- 5.6.2. Product Portfolio: Clinical Trials Software
- 5.6.3. Recent Developments and Future Outlook
- 5.7. IQVIA
- 5.7.1. Company Overview
- 5.7.2. Product Portfolio: Clinical Trials Software
- 5.7.3. Recent Developments and Future Outlook
- 5.8. Medidata
- 5.8.1. Company Overview
- 5.8.2. Product Portfolio: Clinical Trials Software
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Oracle
- 5.9.1. Company Overview
- 5.9.2. Product Portfolio: Clinical Trials Software
- 5.9.3. Recent Developments and Future Outlook
- 5.10. Signant Health
- 5.10.1. Company Overview
- 5.10.2. Product Portfolio: Clinical Trials Software
- 5.10.3. Recent Developments and Future Outlook
- 5.11. Veeva
- 5.11.1. Company Overview
- 5.11.2. Product Portfolio: Clinical Trials Software
- 5.11.3. Recent Developments and Future Outlook
6 CLINICAL TRIALS SOFTWARE DEVELOPERS IN EUROPE: COMPANY PROFILES
- 6.1. Chapter overview
- 6.2. Calyx
- 6.2.1. Company Overview
- 6.2.2. Product Portfolio: Clinical Trials Software
- 6.2.3. Recent Developments and Future Outlook
7 COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Key Parameters and Methodology
- 7.3. Competitiveness Analysis: Companies providing clinical trials software developers
- 7.4. Competitiveness Analysis: Companies providing clinical trials software in North America
- 7.5. Competitiveness Analysis: Companies providing clinical trials software in Europe
- 7.6. Competitiveness Analysis: Companies providing clinical trials software in Asia-Pacific
8 Partnerships and Collaborations
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Clinical Trials Software Market: Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type and Year of Partnership
- 8.4. Geographical Analysis
- 8.4.1. Analysis by Intracontinental and Intercontinental Agreements
- 8.4.2. Analysis by Local and International Agreements
- 8.5. Most Active Players: Analysis by Number of Partnerships
9 MERGERS AND ACQUISITIONS
- 9.1. Chapter Overview
- 9.2. Mergers and Acquisitions Models
- 9.3. Clinical Trials Software Market: Mergers and Acquisitions
- 9.3.1. Analysis by Type of Agreement
- 9.3.2. Analysis by Year of Mergers and Acquisitions
- 9.4. Analysis by Geographical Activity
- 9.4.1. Region-wise Analysis
- 9.4.2. Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- 9.5. Most Active Players: Analysis by Number of Instances Acquisitions and Mergers
10 FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding Instances
- 10.3. Clinical Trials Software Market: Recent Funding Instances
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.4. Most Active Players: Analysis by Number of Funding Instances
- 10.5. Regional Analysis by Amount Invested
- 10.6. Concluding Remarks
11 MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Global Clinical Trials Software Market, 2021-2035
- 11.3.1. Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment
- 11.3.2. Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery
- 11.3.3. Clinical Trials Software Market, 2021-2035: Distribution by Features of Software
- 11.3.4. Clinical Trials Software Market, 2021-2035: Distribution by Geographical Region
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
- 11.3.4.1.1. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Deployment
- 11.3.4.1.2. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Delivery
- 11.3.4.1.3. Clinical Trials Software Market in North America, 2021-2035: Distribution by Features of Software
- 11.3.4.2. Clinical Trials Software Market in Europe, 2021-2035
- 11.3.4.2.1. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Deployment
- 11.3.4.2.2. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Delivery
- 11.3.4.2.3. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Features of Software
- 11.3.4.3. Clinical Trials Software Market in Asia-Pacific, 2021-2035
- 11.3.4.3.1. Clinical Trials Software Market in Asia-Pacific, 2021-2035: Distribution by Type of Deployment
- 11.3.4.3.2. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Type of Delivery
- 11.3.4.3.3. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Features of Software
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
12. CONCLUSION
- 12.1. Chapter Overview
13. Appendix 1: Tabulated Data
14. Appendix 2: List of Companies and Organizations
List of Tables
- Table 4.1 Clinical Trials Software Market: Information on Type of Deployment, Type of Delivery, Type of End-User, Type of features of Software, Type of Trial Design and Type of Technology
- Table 4.2 Clinical Trials Software Market Developers: Information of Year of Establishment, Company Size and Location of Headquarters
- Table 4.3 Clinical Trials Software Market Developers: List of Software and Developers
- Table 6.1 Clinical Trials Software Market: List of Developers in North America
- Table 6.2 Advarra: Company Snapshot
- Table 6.3 Advarra: Product Portfolio
- Table 6.4 Advarra: Recent Developments and Future Outlook
- Table 6.5 ArisGlobal: Company Snapshot
- Table 6.6 ArisGlobal: Product Portfolio
- Table 6.7 ArisGlobal: Recent Developments and Future Outlook
- Table 6.8 AssistRx: Company Snapshot
- Table 6.9 AssistRx: Product Portfolio
- Table 6.10 AssistRx: Recent Developments and Future Outlook
- Table 6.11 Clario: Company Snapshot
- Table 6.12 Clario: Product Portfolio
- Table 6.13 Clario: Recent Developments and Future Outlook
- Table 6.14 IBM: Company Snapshot
- Table 6.15 IBM: Product Portfolio
- Table 6.16 IBM: Recent Developments and Future Outlook
- Table 6.17 IQVIA: Company Snapshot
- Table 6.18 IQVIA: Product Portfolio
- Table 6.19 IQVIA: Recent Developments and Future Outlook
- Table 6.20 Medidata: Company Snapshot
- Table 6.21 Medidata: Product Portfolio
- Table 6.22 Medidata: Recent Developments and Future Outlook
- Table 6.23 Oracle: Company Snapshot
- Table 6.24 Oracle: Product Portfolio
- Table 6.25 Oracle: Recent Developments and Future Outlook
- Table 6.26 Signant Health: Company Snapshot
- Table 6.27 Signant Health: Product Portfolio
- Table 6.28 Signant Health: Recent Developments and Future Outlook
- Table 6.29 Veeva: Company Snapshot
- Table 6.30 Veeva: Product Portfolio
- Table 6.31 Veeva: Recent Developments and Future Outlook
- Table 6.32 Oracle: Company Snapshot
- Table 6.33 Oracle: Product Portfolio
- Table 6.34 Oracle: Recent Developments and Future Outlook
- Table 7.1 Clinical Trials Software Market: List of developers in Europe
- Table 7.2 Calyx: Company Snapshot
- Table 7.3 Calyx: Product Portfolio
- Table 7.4 Calyx: Recent Developments and Future Outlook
- Table 9.1 Clinical Trials Software Market: List of Partnerships and Collaborations, 2016- 2021 (till September)
- Table 10.1 Clinical Trials Software Market: List of Mergers and Acquisitions, 2016-2021 (till September)
- Table 11.1 Clinical Trials Software Market: List of Funding Instances, 2016-2021 (till September)
List of Figures
- Figure 2.1 Executive Summary: Current Market Landscape of Clinical Trials Software Market
- Figure 2.2 Executive Summary: Partnerships and Collaborations
- Figure 2.3 Executive Summary: Funding and Investment
- Figure 2.4 Executive Summary: Market Forecast and Opportunity Analysis
- Figure 4.1 Clinical Trials Software: Distribution by Type of Deployment
- Figure 4.2 Clinical Trials Software: Distribution by Type of Delivery
- Figure 4.3 Clinical Trials Software: Distribution by Type of End-User
- Figure 4.4 Clinical Trials Software: Distribution by Features of Software
- Figure 4.5 Clinical Trials Software: Distribution by Trial Design
- Figure 4.6 Clinical Trials Software: Distribution by Type of Technology
- Figure 4.7 Clinical Trials Software Developers: Distribution by Year of Establishment
- Figure 4.8 Clinical Trials Software Developers: Distribution by Company Size
- Figure 4.9 Clinical Trials Software Developers: Distribution by Geography
- Figure 7.1 Company Competitiveness Analysis: Clinical Trials Software Developers in North America
- Figure 7.2 Company Competitiveness Analysis: Clinical Trials Software Developers in Europe
- Figure 7.3 Company Competitiveness Analysis: Clinical Trials Software Developers in Asia Pacific
- Figure 8.1 Partnerships and Collaborations: Distribution by Year of Partnership
- Figure 8.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 8.3 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Figure 8.4 Partnerships and Collaborations: Geographical Analysis
- Figure 8.5 Partnerships and Collaborations: Distribution by Intracontinental and Intercontinental Agreement
- Figure 8.6 Most Active Players: Distribution by Number of Partnerships
- Figure 9.1 Mergers and Acquisitions: Distribution by Type of Merger and Acquisition
- Figure 9.2 Mergers and Acquisitions: Distribution by Year of Merger and Acquisition
- Figure 9.3 Mergers and Acquisitions: Distribution by Geographical Activity
- Figure 9.4 Mergers and Acquisitions: Continent-wise Distribution
- Figure 9.5 Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- Figure 9.6 Most Active Players: Distribution by Number of Instances
- Figure 10.1 Funding and Investments: Distribution by Year of Investment
- Figure 10.2 Funding and Investments: Distribution by Amount Invested
- Figure 10.3 Funding and Investments: Distribution by Year-wise Trend of Amount Invested, 2016-2021
- Figure 10.4 Funding and Investments: Distribution by Type of Funding
- Figure 10.5 Funding and Investments: Distribution of Amount Invested by Type of Funding
- Figure 10.6 Most Active Players: Distribution by Amount invested
- Figure 10.7 Funding and Investments: Geographical Distribution by Amount Invested, 2016-2021
- Figure 11.1 Clinical Trials Software Market, 2021-2035 (USD Million)
- Figure 11.2 Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment (USD Million)
- Figure 11.3 Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery (USD Million)
- Figure 11.4 Clinical Trials Software Market, 2021-2035: Distribution by Features of Software (USD Million)






