 |
市場調查報告書
商品編碼
1852108
空膠囊:市場佔有率分析、產業趨勢、統計數據、成長預測(2025-2030 年)Empty Capsules - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
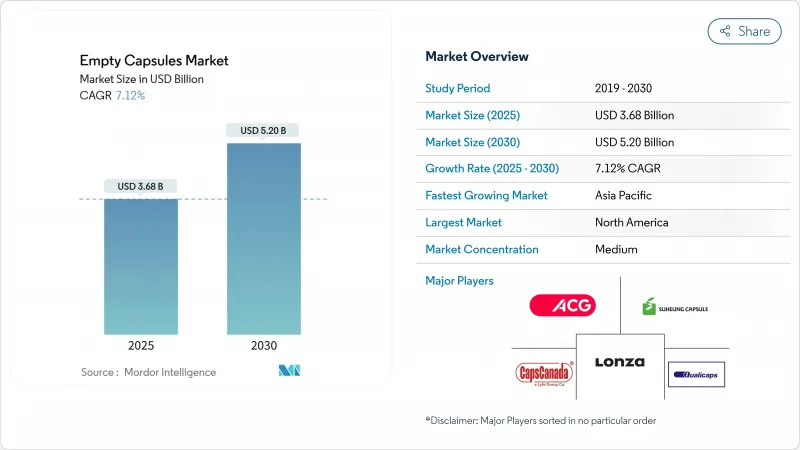
預計到 2025 年,空膠囊市場規模將達到 36.8 億美元,到 2030 年將達到 52 億美元,年複合成長率為 7.12%。
成長的驅動力來自藥品產量的擴大、膠囊劑型治療方法的廣泛應用以及高速填充設備的快速改進。尤其是在北美和歐洲,對單劑量包裝的需求不斷成長,這需要符合嚴格監管要求和以患者為中心的優質膠囊。整合機器視覺和物聯網感測器的技術升級提高了一次產量比率率,降低了單位成本,並促進了產能擴張。同時,膳食補充品品牌也開始採用膠囊來保護敏感成分,這為市場需求增添了新的多樣性。隨著主要供應商在印度和中國擴大產能,同時探索剝離業務和成立合資企業以期獲得更清晰的策略方向,市場競爭日益激烈。
全球空膠囊市場趨勢與洞察
增加藥品產量
人口老化和保險覆蓋範圍的擴大正在推動藥品生產擴張,從而維持對高精度空膠囊市場供應鏈的需求。龍沙公司斥資8,500萬瑞士法郎進行升級改造,使其年產能增加300億粒,體現了其致力於透過擴大產能來確保供應連續性的承諾。連續生產線需要原料的均一性和即時品質數據,這對高階膠囊的生產至關重要。生技藥品產量的增加推動了對無污染膠囊殼的需求。靈活的生產模式,例如工廠持有更多安全庫存以實現快速換線,進一步推動了各地區膠囊的消費量成長。
膳食補充劑攝取量增加
預防性健康措施正促使消費者轉向益生菌、適應原和定製配方產品,而這些產品通常採用膠囊包裝以保護敏感的活性成分。配方生產商正在摒棄動物性成分,轉而選擇植物來源丙甲纖維素(HPMC)外殼,以實現「潔淨標示」的宣傳。這一趨勢在美國和德國尤為明顯,益生菌膠囊的銷售量正呈現兩位數的成長。小批量生產以滿足個性化營養需求,也為特定規格的膠囊創造了新的訂單。品牌擁有者也開始使用透明或彩色外殼,並採用透明標籤,進一步強化了膠囊作為高階遞送載體的形象。
明膠原料供應不穩定
牛和豬的供應趨緊,以及藥用級明膠可能被徵收10-25%的進口關稅,將給空膠囊市場帶來短期成本壓力。中國工廠的季節性停工將進一步加劇供應短缺,使依賴單一供應商合約的公司面臨風險。製藥採購商正採取雙重採購和預購策略來應對,但這種策略會消耗大量營運資金。天氣導致的畜牧業中斷可能會持續,這將促使配方師轉向植物來源膠囊殼,儘管其單位成本更高。因此,明膠持續不穩定可能會加速羥丙基(HPMC)膠囊的普及。
細分市場分析
2024年,明膠膠囊殼佔了空膠囊市場84.34%的佔有率。然而,非明膠膠囊預計將以10.32%的複合年成長率成長,這表明在潔淨標示偏好和多宗教合規要求的推動下,市場結構正在轉變。硬明膠膠囊在大容量口服固體製劑中佔據主導地位,而軟明膠膠囊則用於脂溶性製劑。纖維素化學的創新使得羥丙基甲基纖維素(HPMC)膠囊殼具備了以往只有明膠才能擁有的溶解性和機械性能。普魯蘭膠膠囊和澱粉膠囊則佔據了高階市場,它們宣稱具有優異的氧氣阻隔性能或特定的素食相容性。
HPMC的應用在營養補充劑領域中成長最為迅速,尤其適用於封裝對水分敏感的益生菌和草本萃取物。生產商正透過最佳化乾燥過程中的水分含量來克服以往HPMC易碎的問題。普魯蘭膠多醣優異的耐氧性使其成為高效價植物萃取物的理想選擇。新型腸溶HPMC膠囊,例如Enprotect,無需二次包衣即可實現98%的緩衝劑釋放效率,從而減少了加工步驟,並檢驗了其高階定位。澱粉類HPMC膠囊目前仍僅限於對成本較為敏感的領域,但如果其轉化率能夠進一步提高,則有望從中受益。
到2024年,即時釋膠囊殼將佔據空膠囊市場72.45%的佔有率,這主要得益於其成熟的標準。然而,隨著製藥公司致力於開發每日一次給藥方案以提高患者用藥依從性,緩釋性膠囊預計將以9.84%的複合年成長率成長。延遲釋放膠囊正透過聚合物層層包裹技術不斷發展,以實現特異性溶出,填補了酸不穩定原料藥這一雖小但具有戰略意義的市場空白。順序包衣技術能夠實現更均勻的聚合物分佈,從而提高批次均一性並簡化監管申報流程。
這種雙層殼將速釋和緩釋特性結合於一體,無需片劑-膠囊混合製劑。其pH值非依賴性基質能夠適應不同患者胃部環境的差異。連續生產線內建的即時分析系統使操作人員能夠直接控制包衣增重,從而減少偏差。隨著數位療法將藥物釋放數據整合到患者資訊平台中,預計在預測期內,對客製化釋放曲線的需求將日益成長。
區域分析
北美在空膠囊市場保持主導,2024年市佔率達38.54%,這得益於先進的GMP設施和FDA的嚴格監管,後者尤其注重文件記錄和批次一致性。美國的連續生產測試要求供應商滿足嚴格的規格範圍和即時放行測試。加拿大學名藥的生產以及墨西哥的契約製造基地也增加了該地區對膠囊的需求。能夠獲得FDA、加拿大衛生署和USP認證的工廠正在獲得優先供應商地位。
到2030年,亞太地區將以8.72%的複合年成長率保持最高成長,再形成全球藥品流通格局。中國正在升級其醫藥基礎設施,但一項針對低成本膠囊出口的反傾銷調查迫使其調整籌資策略。印度正利用其成本優勢和熟練勞動力,投資2.5億盧比擴大天然膠囊業務,顯示對出口交易充滿信心。日本則瞄準老年人專用製劑,這些製劑需要易於吞嚥的膠囊殼,因此傾向於較小的膠囊尺寸和增強潤滑劑。隨著全民健保擴大了口服藥物的覆蓋範圍,東南亞國家也正在跟進。
歐洲依然是一個成熟且充滿創新活力的市場。德國的生物技術中心需要先進的膠囊技術來生產特殊藥品,而英國脫歐後的政策調整也刺激了英國的投資。歐盟的環境指令鼓勵轉向植物來源膠囊殼,羥丙基甲基纖維素(HPMC)的應用也日益普及。生產商正大力宣傳其低碳足跡和無溶劑生產流程,以契合企業的永續性目標。在歐盟範圍內,個人化醫療試點計畫正在使用電子處方平台,這使得能夠快速小批量交付的膠囊供應商獲得了競爭優勢。
其他福利:
- Excel格式的市場預測(ME)表
- 3個月的分析師支持
目錄
第1章 引言
- 研究假設和市場定義
- 調查範圍
第2章調查方法
第3章執行摘要
第4章 市場情勢
- 市場概覽
- 市場促進因素
- 增加藥品產量
- 膳食補充劑攝取量增加
- 膠囊填充技術的進步
- 轉向獨立劑量包裝
- 整合數位健康技術
- 擴大常規生產基礎設施
- 市場限制
- 明膠原料供應的波動性
- 嚴格遵守宗教和飲食規定
- 藥用級羥丙基甲基纖維素(HPMC)供應有限。
- 氣候相關穩定性挑戰對供應鏈的影響
- 監管環境
- 波特五力模型
- 新進入者的威脅
- 買方的議價能力
- 供應商的議價能力
- 替代品的威脅
- 競爭對手之間的競爭
第5章 市場規模與成長預測
- 依產品
- 明膠膠囊
- 硬明膠膠囊
- 軟膠囊
- 非明膠膠囊
- HPMC膠囊
- 普魯蘭膠膠囊
- 澱粉基膠囊
- 明膠膠囊
- 功能
- 即時釋膠囊
- 延遲釋放膠囊
- 緩釋性膠囊
- 治療用途
- 抗生素和抗菌劑
- 維生素和營養補充劑
- 制酸劑和腸道調節劑
- 心血管治療
- 其他治療用途
- 最終用戶
- 製藥業
- 營養保健品產業
- 化妝品和個人護理
- 研究和學術機構
- 按地區
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 俄羅斯
- 其他歐洲地區
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 亞太其他地區
- 中東和非洲
- GCC
- 南非
- 其他中東和非洲地區
- 南美洲
- 巴西
- 阿根廷
- 其他南美洲國家
- 北美洲
第6章 競爭情勢
- 市場集中度
- 市佔率分析
- 公司簡介
- ACG Worldwide
- Lonza Group(Capsugel)
- Qualicaps
- Suheung Capsule Co. Ltd
- CapsCanada Corporation
- Bright Pharma Caps Inc.
- Medi-Caps Ltd
- HealthCaps India Ltd
- Sunil Healthcare Ltd
- Fujifilm Corp.(Fujicaps)
- Roxlor LLC
- Farmacapsulas SA
- Patheon(Thermo Fisher)
- Sirio Pharma Co., Ltd.
- Er-Kang Pharmaceutical Co. Ltd
- Qingdao Yiqing Medicinal Capsules Co. Ltd
- Shanxi Guangsheng Medicinal Capsules Co. Ltd
- Shanxi JC Biological Technology Co. Ltd
- Natural Capsules Ltd
- Zhejiang Huangyan Gelatin Capsule Co. Ltd
- Zhejiang Ruixin Capsules Co. Ltd
第7章 市場機會與未來展望
The empty capsules market size is valued at USD 3.68 billion in 2025 and is forecast to reach USD 5.20 billion by 2030, expanding at a 7.12% CAGR.

Growth springs from larger pharmaceutical production runs, wider acceptance of capsule-based therapies and rapid improvements in high-speed filling equipment. Rising demand for personalized dose packaging, especially in North America and Europe, lifts premium capsule grades that meet strict regulatory and patient-centric requirements. Technology upgrades that integrate machine vision and IoT sensors are raising first-pass yield rates and lowering unit costs, encouraging producers to scale capacity. Simultaneously, nutraceutical brands are shifting to capsules to protect delicate ingredients, which adds a fresh layer of demand diversity. Competitive intensity is sharpening as leading suppliers expand output in India and China while exploring divestitures or joint ventures that promise sharper strategic focus.
Global Empty Capsules Market Trends and Insights
Growing Pharmaceutical Manufacturing Volume
Expanding drug output, propelled by aging populations and wider insurance coverage, creates sustained demand for high-precision empty capsules market supply chains. Lonza's CHF 85 million upgrade that adds 30 billion units a year shows how producers scale capacity to secure continuity of supply. Continuous production lines need feedstock uniformity and real-time quality data, which favors premium capsule grades. Heightened biologics output intensifies requirements for contamination-free shells. Flexible manufacturing models, where plants hold larger safety stocks to enable rapid changeovers, further lift unit consumption across regions.
Rising Nutraceutical Consumption
Preventive-health habits are steering consumers toward probiotics, adaptogens and bespoke blends that rely on capsules to protect sensitive actives. Formulators pick plant-based HPMC shells for clean-label claims because they avoid animal derivatives. Double-digit sales growth of probiotic capsules in the United States and Germany demonstrates the trend. Smaller batch runs for personalized nutrition create fresh orders for niche capsule sizes. Brand owners also use clear or tinted shells to signal transparent labeling, which reinforces the capsule format as a premium delivery vehicle.
Volatility in Gelatin Raw-Material Supply
Tighter bovine and porcine supply and potential 10-25% import tariffs on pharmaceutical-grade gelatin raise short-term cost pressure on the empty capsules market. Seasonal plant shutdowns in China compound scarcity, exposing firms that rely on single-source contracts. Pharmaceutical buyers respond by dual-sourcing and pre-booking volumes, but that strategy ties up working capital. Climate-driven livestock disruptions may persist, nudging formulators toward plant-based shells despite higher unit prices. Broader adoption of HPMC capsules could therefore accelerate if gelatin volatility persists.
Other drivers and restraints analyzed in the detailed report include:
- Advancements in Capsule Filling Technology
- Shift Toward Personalized Dose Packaging
- Stringent Religious and Dietary Compliance
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Gelatin shells retained 84.34% of empty capsules market share in 2024, thanks to entrenched cost advantages and proven functionality. Yet non-gelatin capsules are moving at a 10.32% CAGR, signaling a structural pivot driven by clean-label preferences and multi-faith compliance requirements. Hard gelatin variants dominate high-volume oral solids, while soft gelatin capsules support lipid-soluble formulations. Innovation in cellulose chemistry equips HPMC shells with dissolution and mechanical properties once exclusive to gelatin. Pullulan and starch capsules occupy premium niches where oxygen barrier performance or specific vegetarian claims hold sway.
HPMC adoption rises fastest in nutraceutical lines that encapsulate moisture-sensitive probiotics or herbal extracts. Producers overcome previous brittleness by optimizing moisture content during drying. Pullulan's superior oxygen resistance makes it ideal for high-potency botanicals. Emerging enteric HPMC capsules such as Enprotect reach 98% buffer-conditioned release efficiency without secondary coating, cutting process steps and validating the premium positioning. Starch variants remain limited to cost-sensitive segments but stand to benefit if conversion economics further improve.
Immediate-release shells held 72.45% share of the empty capsules market size in 2024, favored for their established compendial standards. However, sustained-release uses are growing at 9.84% CAGR as pharma companies chase once-daily regimens that lift adherence. Delayed-release capsules fill a smaller yet strategic niche for acid-labile APIs, evolving through polymer layering that triggers site-specific dissolution. Continuous coating methods yield tighter polymer distribution, enhancing batch uniformity and easing regulatory filings.
Bi-layer shells merge immediate and sustained profiles in a single unit, eliminating the need for tablet-capsule hybrids. pH-independent matrices counter patient-to-patient variability in gastric conditions. Real-time analytics embedded in continuous lines give operators direct control over coating weight gain, reducing deviations. As digital therapeutics integrate drug release data into patient dashboards, demand for bespoke release profiles is poised to strengthen over the forecast horizon.
The Empty Capsules Market Report is Segmented by Product (Gelatin Capsules and Non-Gelatin Capsules), Functionality (Immediate-Release Capsules, and More), Therapeutic Application (Antibiotic & Antibacterial, and More), End User (Pharmaceutical Industry, and More), Geography (North America, Europe, Asia-Pacific, The Middle East and Africa, and South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained leadership with a 38.54% stake in the empty capsules market in 2024, backed by advanced GMP facilities and FDA oversight that prizes documentation and batch consistency. Continuous manufacturing pilots in the United States push suppliers to meet tight specification ranges and real-time release testing. Canada's generics production and Mexico's contract-manufacturing base add depth to regional capsule demand. Plants that can certify for FDA, Health Canada and USP standards thus win preferred-supplier status.
Asia-Pacific records the highest 8.72% CAGR through 2030 and is set to re-shape global volume flows. China upgrades its pharmaceutical infrastructure while antidumping probes into low-cost capsule exports force realignment of sourcing strategies. India leverages cost advantages and a skilled workforce; Natural Capsules' INR 250 million expansion indicates confidence in export contracts. Japan targets geriatric formulations that demand easy-to-swallow shells, favoring smaller sizes and enhanced glidants. Southeast Asian economies follow suit as universal healthcare programs expand access to oral therapies.
Europe maintains a mature yet innovative landscape. German biotechnology hubs require advanced capsule technologies for specialty drugs, while post-Brexit policy tweaks stimulate United Kingdom-based investments. EU environmental directives encourage a pivot to plant-based shells, with HPMC uptake gathering momentum. Producers market low-carbon footprints and solvent-free processes to align with corporate sustainability targets. Across the bloc, personalized medicine pilots tap into e-prescription platforms, and capsule suppliers that can provide rapid small-lot deliveries gain competitive edges.
- ACG Worldwide
- Lonza Group
- Qualicaps
- Suheung Capsule
- CapsCanada Corporation
- Bright Pharma Caps
- Medi-Caps
- HealthCaps India Ltd
- Sunil Healthcare Ltd
- Fujifilm Corp. (Fujicaps)
- Roxlor LLC
- Farmacapsulas S.A.
- Patheon (Thermo Fisher)
- Sirio Pharma Co., Ltd.
- Er-Kang Pharmaceutical Co. Ltd
- Qingdao Yiqing Medicinal Capsules
- Shanxi Guangsheng Medicinal Capsules
- Shanxi JC Biological Technology
- Natural Capsules Ltd
- Zhejiang Huangyan Gelatin Capsule Co. Ltd
- Zhejiang Ruixin Capsules Co. Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Pharmaceutical Manufacturing Volume
- 4.2.2 Rising Nutraceutical Consumption
- 4.2.3 Advancements in Capsule Filling Technology
- 4.2.4 Shift Toward Personalized Dose Packaging
- 4.2.5 Integration of Digital Health Technologies
- 4.2.6 Expansion of Continuous Manufacturing Infrastructure
- 4.3 Market Restraints
- 4.3.1 Volatility In Gelatin Raw Material Supply
- 4.3.2 Stringent Religious and Dietary Compliance
- 4.3.3 Limited Availability of Pharmaceutical-Grade HPMC
- 4.3.4 Climate-Induced Stability Challenges In Supply Chain
- 4.4 Regulatory Landscape
- 4.5 Porter's Five Forces
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitutes
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product
- 5.1.1 Gelatin Capsules
- 5.1.1.1 Hard Gelatin Capsules
- 5.1.1.2 Soft Gelatin Capsules
- 5.1.2 Non-Gelatin Capsules
- 5.1.2.1 HPMC Capsules
- 5.1.2.2 Pullulan Capsules
- 5.1.2.3 Starch-Based Capsules
- 5.1.1 Gelatin Capsules
- 5.2 By Functionality
- 5.2.1 Immediate-Release Capsules
- 5.2.2 Delayed-Release Capsules
- 5.2.3 Sustained/Extended-Release Capsules
- 5.3 By Therapeutic Application
- 5.3.1 Antibiotic & Antibacterial
- 5.3.2 Vitamins & Dietary Supplements
- 5.3.3 Antacid & Antiflatulent
- 5.3.4 Cardiovascular Therapy
- 5.3.5 Other Therapeutic Applications
- 5.4 By End User
- 5.4.1 Pharmaceutical Industry
- 5.4.2 Nutraceutical Industry
- 5.4.3 Cosmetic & Personal-Care
- 5.4.4 Research & Academic Laboratories
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Russia
- 5.5.2.7 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.3.1 ACG Worldwide
- 6.3.2 Lonza Group (Capsugel)
- 6.3.3 Qualicaps
- 6.3.4 Suheung Capsule Co. Ltd
- 6.3.5 CapsCanada Corporation
- 6.3.6 Bright Pharma Caps Inc.
- 6.3.7 Medi-Caps Ltd
- 6.3.8 HealthCaps India Ltd
- 6.3.9 Sunil Healthcare Ltd
- 6.3.10 Fujifilm Corp. (Fujicaps)
- 6.3.11 Roxlor LLC
- 6.3.12 Farmacapsulas S.A.
- 6.3.13 Patheon (Thermo Fisher)
- 6.3.14 Sirio Pharma Co., Ltd.
- 6.3.15 Er-Kang Pharmaceutical Co. Ltd
- 6.3.16 Qingdao Yiqing Medicinal Capsules Co. Ltd
- 6.3.17 Shanxi Guangsheng Medicinal Capsules Co. Ltd
- 6.3.18 Shanxi JC Biological Technology Co. Ltd
- 6.3.19 Natural Capsules Ltd
- 6.3.20 Zhejiang Huangyan Gelatin Capsule Co. Ltd
- 6.3.21 Zhejiang Ruixin Capsules Co. Ltd
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment











