 |
市場調查報告書
商品編碼
1851453
藥品包裝:市場佔有率分析、產業趨勢、統計數據和成長預測(2025-2030 年)Pharmaceutical Packaging - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
預計到 2025 年,藥品包裝市場規模將達到 1,547.8 億美元,到 2030 年將達到 2,074.2 億美元,年複合成長率為 6.03%。
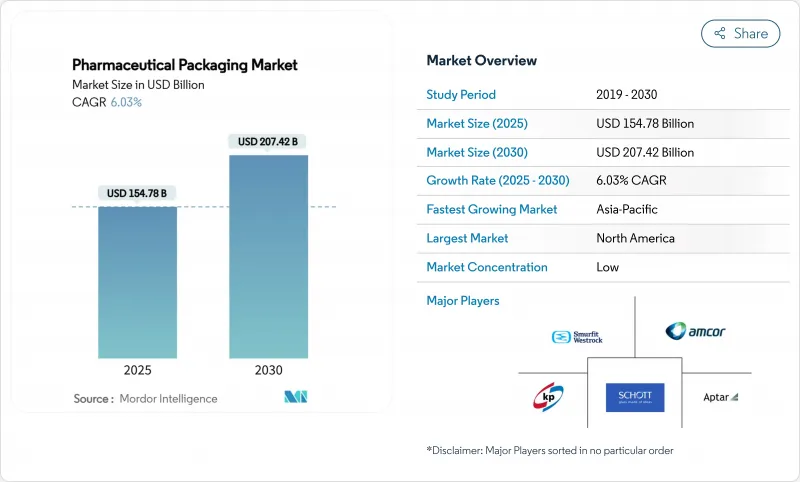
未來五年,生技藥品產量不斷成長、全球可追溯性法規日益嚴格以及永續性推進,將繼續推動資本流入新的灌裝生產線、高阻隔材料和循環經濟型設計領域。隨著基因和細胞療法達到商業性規模,對能夠滿足更小批量、個人化治療需求的靈活包裝產能的需求也將隨之成長。亞太地區8.96%的複合年成長率反映了國內藥品產量的成長和醫療覆蓋範圍的擴大。材料策略正處於不斷變化之中,塑膠仍佔據市場主導地位,但隨著歐盟和美國PFAS法規即將實施,生物基聚合物、無鋁泡殼和消費後回收薄膜正迅速從試點生產轉向量產。同時,聚乙烯、聚丙烯和PET價格的波動正在收窄利潤淨利率,促使大型加工商簽訂長期供應商合約並進行垂直整合。
全球醫藥包裝市場趨勢與洞察
人口老化和慢性病流行
人口平均年齡的成長將推動長期用藥需求的增加,從而支撐對日曆式泡殼、大尺寸標籤和單手開啟管瓶的穩定需求,這些包裝有助於提高行動不便患者的用藥依從性。德國2024年的疫苗接種計畫調整,肺炎鏈球菌疫苗接種量增加23%,B族腦膜炎雙球菌疫苗接種量增加52%,顯示老年人對預防保健的接受度正在提高。包裝供應商正在積極回應,推出可記錄開啟事件並將依從性資料傳輸給醫療團隊的智慧包裝。隨著支付方將報銷與實際療效掛鉤,智慧封口和支援NFC功能的紙匣的需求將進一步成長。
擴大生技藥品和注射產品線
預填充式注射器對於新生物製藥的上市至關重要,因為它們簡化了患者自行給藥流程,最大限度地降低了污染風險,並減少了灌裝結束時的廢棄物。 BD 的 iDFill™ 注射器整合了 RFID 技術,可即時識別;而 Neopak™ XtraFlow™ 設計可容納以往只能管瓶包裝的黏稠製劑。 GMP 附錄 1 的修訂正在加速市場對即用型玻璃管和聚合物容器的需求,這些容器無需清洗和除熱原步驟,有助於 CDMO 在無需新建無塵室的情況下擴大產能。
石油衍生樹脂的價格波動
供應中斷和不可抗力事件導致PET價格在2024年6月上漲1.1%,進一步擠壓了加工業者本已微薄的利潤空間。醫藥接觸材料的規格限制了快速更換等級的能力,迫使許多加工商要么承擔更高的成本,要么重新談判長期合約。瓦楞紙板供應商也面臨纖維成本上漲的困境,並宣布將於2025年1月將價格上調70美元/噸。
細分市場分析
到2024年,塑膠將佔藥品包裝市場45.64%的佔有率,其中以高密度聚乙烯(HDPE)瓶、聚丙烯(PP)瓶蓋和聚對苯二甲酸乙二醇酯(PET)泡殼包裝為主,這些產品在成本和阻隔性能之間取得了良好的平衡。然而,隨著品牌所有者推行循環經濟目標,該領域的成長速度將會放緩。在塑膠領域,由於抗碎裂環烯烴材料的出現,聚丙烯(PP)注射器的市場規模正在穩定成長。玻璃仍然是光敏性和濕敏性生技藥品包裝的必備材料,而i型硼矽酸管瓶儘管重量較重且存在破碎風險,但正逐漸成為細胞毒性填充劑的主流包裝。金屬則在氣霧劑和植入式醫療器材領域發揮獨特的作用。
生物基樹脂、再生PET中阻隔膜以及紙質藥瓶(例如阿勒格尼健康網路的TallyTube試點計畫)正蓬勃發展。研發供應商在產品大規模上市前會考慮保存期限保證、萃取物特性以及生產線切換成本,而早期採用這些產品的醫院則會在供應商審核中加入永續性評分,以贏得採購競標。
區域分析
到2024年,北美將佔全球藥品包裝市場35.32%的佔有率,其中1,600億美元的投資將用於提升生技藥品產能和增強國內供應韌性。 《藥品供應鏈安全法案》(DSCSA)的序列化法規將促進編碼設備的升級,而全氟烷基和多氟烷基物質(PFAS)的早期淘汰將推動聚合物再製造。德國2024年的藥品產量將下降1.5%,但mRNA、基因和放射性藥物研發投入的持續成長將推動高阻隔包裝的需求。隨著生產者責任費的擴大,可回收包裝形式將獲得獎勵,預計全部區域的包裝收入將從2024年的1530億歐元增加到2029年的1860億歐元。
亞太地區將達到最高的複合年成長率,達到8.96%。中國和印度將擴大原料藥生產,吸引合約研發生產機構(CDMO)的投資,這些機構需要在更嚴格的供應鏈安全法規下採購本地包裝。日本藥品醫療器材管理局(PMDA)的嚴格標準與歐盟的無菌升級標準相呼應,將迫使企業儘早採用符合附件一標準的隔離器。地緣政治變化帶來風險:中國的反間諜法可能會使與包裝序列化合作夥伴的技術轉移和資料共用變得複雜。在全部區域,醫療保健的擴張和分散式臨床模式正在推動可郵寄、溫控運輸包裝的需求。
其他福利:
- Excel格式的市場預測(ME)表
- 3個月的分析師支持
目錄
第1章 引言
- 研究假設和市場定義
- 調查範圍
第2章調查方法
第3章執行摘要
第4章 市場情勢
- 市場概覽
- 市場促進因素
- 人口老化和慢性病流行
- 擴大生技藥品和注射產品線
- 永續性主導的材料替代
- 強制性數位可追溯性(例如,DSCSA、歐盟FMD)
- 人工智慧賦能的自適應灌裝生產線(報道不足)
- 居家/分散式臨床試驗數量增加,需要即用型試劑盒(未充分報告)
- 市場限制
- 石油衍生樹脂價格波動
- 資本密集的無菌和驗證要求
- 歐盟和美國即將推出 PFAS/氟聚合物法規(通報不足)
- 供應鏈分析
- 監管環境
- 技術展望
- 波特五力分析
- 供應商的議價能力
- 買方的議價能力
- 新進入者的威脅
- 替代品的威脅
- 競爭程度
第5章 市場規模與成長預測
- 材料
- 塑膠
- 高密度聚苯乙烯
- 低密度聚乙烯和線性低密度聚乙烯
- PET
- 其他塑膠
- 玻璃
- I型硼矽酸
- II 型處理鈉石灰
- 第三型鈉石灰
- 金屬
- 紙和紙板
- 生物聚合物和其他材料
- 塑膠
- 按包裝級別
- 初級包裝
- 瓶子
- 預填充式注射器
- 管瓶和安瓿
- 泡殼包裝
- 二級包裝
- 紙箱和紙套
- 標籤和插頁
- 三級包裝
- 紙板運輸箱
- 托盤和防護系統
- 初級包裝
- 依產品類型
- 瓶子
- 預填充式注射器
- 管瓶和安瓿
- 泡殼包裝
- 蓋子與封口裝置
- 管狀和袋狀
- 其他產品類型
- 按地區
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 俄羅斯
- 其他歐洲地區
- 亞太地區
- 中國
- 印度
- 日本
- 韓國
- 澳洲和紐西蘭
- 亞太其他地區
- 中東和非洲
- 中東
- 阿拉伯聯合大公國
- 沙烏地阿拉伯
- 土耳其
- 其他中東地區
- 非洲
- 南非
- 奈及利亞
- 埃及
- 其他非洲地區
- 南美洲
- 巴西
- 阿根廷
- 其他南美洲
- 北美洲
第6章 競爭情勢
- 市場集中度
- 策略趨勢
- 市佔率分析
- 公司簡介
- Amcor plc
- Gerresheimer AG
- Schott AG
- West Pharmaceutical Services Inc.
- AptarGroup Inc.
- Smurfit WestRock
- Becton, Dickinson & Company
- Catalent Inc.
- CCL Industries Inc.
- Klockner Pentaplast Group
- Nipro Corporation
- Vetter Pharma International GmbH
- McKesson Corporation
- FlexiTuff International Ltd.
- WL Gore & Associates Inc.
- Stevanato Group
- Corning Incorporated
- Owen-Illinois Inc.
- SGD Pharma
第7章 市場機會與未來展望
The pharmaceutical packaging market size reached USD 154.78 billion in 2025 and is forecast to rise to USD 207.42 billion by 2030, advancing at a 6.03% CAGR.

Over the next five years, escalating biologics output, stricter global traceability rules, and widespread sustainability targets will keep capital flowing into new fill-finish lines, high-barrier materials, and circular-ready designs. Demand for flexible pack volumes that match smaller, personalized therapy batches will expand as gene and cell therapies reach commercial scale. North America remains the largest regional contributor, supported by DSCSA-driven serialisation, while Asia-Pacific's sizeable 8.96% CAGR reflects rising domestic drug production and broadening health coverage.Material strategies are in flux: plastics still dominate yet bio-based polymers, aluminium-free blisters, and post-consumer-recycled films move quickly from pilot to production as EU and US PFAS curbs near enforcement. Meanwhile, price swings in polyethylene, polypropylene, and PET keep margins tight, encouraging longer supplier contracts and vertical integration by larger converters.
Global Pharmaceutical Packaging Market Trends and Insights
Aging population and chronic disease prevalence
Rising median ages push long-term therapy volumes higher, underpinning consistent demand for calendar blisters, large-print labels, and one-hand-open vials that aid adherence among patients with reduced dexterity. Germany's 2024 vaccination shifts, with pneumococcal doses up 23% and meningococcal B up 52%, illustrate broader preventive-care uptake in seniors . Packaging suppliers respond with connected packs that log opening events and forward adherence data to care teams. Growth in smart closures and NFC-enabled cartons will intensify as payers link reimbursement to real-world outcomes.
Biologics and injectable pipeline expansion
Prefilled syringes sit at the core of new biologic launches because they simplify self-administration, minimise contamination risks, and reduce waste during fill-finish. BD's iDFill(TM) syringe embeds RFID for instant verification, while its Neopak(TM) XtraFlow(TM) design handles viscous formulations that were once vial-only. GMP Annex 1 revisions accelerate demand for ready-to-use glass tubing and polymer containers that bypass washing and depyrogenation steps, helping CDMOs scale capacity without constructing new cleanrooms.
Petro-derivative resin price volatility
Supply disruptions and force majeure events lifted PET prices by 1.1% in June 2024, shrinking already tight converter margins. Pharmaceutical contact material specs restrict rapid grade switches, forcing many converters to absorb cost spikes or renegotiate long contracts. Corrugated shippers also face higher fibre costs, with a USD 70 per-ton increase announced for January 2025.
Other drivers and restraints analyzed in the detailed report include:
- Sustainability-driven material substitution
- Digital traceability mandates (DSCSA, EU-FMD)
- Capital-intensive sterility and validation requirements
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Plastics retained 45.64% of pharmaceutical packaging market share in 2024, anchored by HDPE bottles, PP closures, and PET blisters that balance cost and barrier needs. Yet the segment's growth moderates as brand owners court circularity objectives. Within plastics, the pharmaceutical packaging market size for PP-based syringes is rising steadily thanks to break-resistant cyclic olefin options. Glass remains indispensable for light- and moisture-sensitive biologics; Type I borosilicate vials dominate cytotoxic fills despite higher weight and shatter risk. Metals hold niche aerosol and implantable device roles.
Momentum gathers around bio-attributed resins, recycled PET mid-barrier webs, and paper-based pill bottles such as Allegheny Health Network's Tully Tube pilot. Developers weigh shelf-life assurance, extractables profiles, and line changeover costs before wide release, yet early adopters win procurement tenders from hospitals adding sustainability scoring to vendor audits.
The Pharmaceutical Plastic Packaging Market Report is Segmented by Raw Material (Polypropylene, and More), Product Type (Bottles and Solid Containers, Vials and Ampoules, and More), Packaging Format (Rigid, Flexible), Route of Drug Delivery (Oral, Parenteral/Injectable, and More), End-User (Pharma Manufacturers, and More), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America contributed 35.32% of pharmaceutical packaging market share in 2024 as investments worth USD 160 billion target biologics capacity and domestic supply resilience. DSCSA serialisation stipulations stimulate coding machinery upgrades, and early PFAS phase-outs drive polymer reformulation. Europe balances stringent green-deal rules with high-cost energy inputs; Germany saw 1.5% output decline in 2024 yet sustained R&D outlays in mRNA, gene, and radiopharma therapies demanding high-barrier packs. Region-wide packaging revenue is expected to rise from EUR 153 billion in 2024 to EUR 186 billion by 2029 as extended-producer-responsibility fees reward recyclable formats.
Asia-Pacific records the strongest 8.96% CAGR. China and India expand API output and attract CDMO investments that need local pack sourcing under tighter supply-security rules. Japan's stringent PMDA standards enforce early adoption of Annex 1 aligned isolators, mirroring EU sterility upgrades. Geopolitical shifts introduce risk: China's anti-espionage laws potentially complicate tech transfer and data sharing for pack serialisation partners. Across the region, national healthcare expansions and decentralised clinical models spur demand for mail-ready temperature-controlled shippers.
- Amcor plc
- Gerresheimer AG
- Schott AG
- West Pharmaceutical Services Inc.
- AptarGroup Inc.
- Smurfit WestRock
- Becton, Dickinson & Company
- Catalent Inc.
- CCL Industries Inc.
- Klockner Pentaplast Group
- Nipro Corporation
- Vetter Pharma International GmbH
- McKesson Corporation
- FlexiTuff International Ltd.
- W. L. Gore & Associates Inc.
- Stevanato Group
- Corning Incorporated
- Owen-Illinois Inc.
- SGD Pharma
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Ageing population and chronic disease prevalence
- 4.2.2 Biologics and injectable pipeline expansion
- 4.2.3 Sustainability-driven material substitution
- 4.2.4 Digital traceability mandates (e.g., DSCSA, EU-FMD)
- 4.2.5 AI-enabled adaptive fill-finish lines (under-reported)
- 4.2.6 Rise of at-home/?decentralised trials needing mail-ready packs (under-reported)
- 4.3 Market Restraints
- 4.3.1 Petro-derivative resin price volatility
- 4.3.2 Capital-intensive sterility and validation requirements
- 4.3.3 Looming PFAS/fluoropolymer restrictions in EU and US (under-reported)
- 4.4 Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Degree of Competition
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Material
- 5.1.1 Plastics
- 5.1.1.1 HDPE
- 5.1.1.2 LDPE and LLDPE
- 5.1.1.3 PET
- 5.1.1.4 Other Plastics
- 5.1.2 Glass
- 5.1.2.1 Type I Borosilicate
- 5.1.2.2 Type II Treated Soda-lime
- 5.1.2.3 Type III Soda-lime
- 5.1.3 Metal
- 5.1.4 Paper and Paperboard
- 5.1.5 Biopolymers and Other Materials
- 5.1.1 Plastics
- 5.2 By Packaging Level
- 5.2.1 Primary Packaging
- 5.2.1.1 Bottles
- 5.2.1.2 Prefilled Syringes
- 5.2.1.3 Vials and Ampoules
- 5.2.1.4 Blister Packs
- 5.2.2 Secondary Packaging
- 5.2.2.1 Cartons and Sleeves
- 5.2.2.2 Labels and Inserts
- 5.2.3 Tertiary Packaging
- 5.2.3.1 Corrugated Shippers
- 5.2.3.2 Pallets and Protective Systems
- 5.2.1 Primary Packaging
- 5.3 By Product Type
- 5.3.1 Bottles
- 5.3.2 Prefilled Syringes
- 5.3.3 Vials and Ampoules
- 5.3.4 Blister Packs
- 5.3.5 Caps and Closures
- 5.3.6 Tubes and Pouches
- 5.3.7 Other Product Types
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Russia
- 5.4.2.7 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 India
- 5.4.3.3 Japan
- 5.4.3.4 South Korea
- 5.4.3.5 Australia and New Zealand
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 Middle East
- 5.4.4.1.1 United Arab Emirates
- 5.4.4.1.2 Saudi Arabia
- 5.4.4.1.3 Turkey
- 5.4.4.1.4 Rest of Middle East
- 5.4.4.2 Africa
- 5.4.4.2.1 South Africa
- 5.4.4.2.2 Nigeria
- 5.4.4.2.3 Egypt
- 5.4.4.2.4 Rest of Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 Amcor plc
- 6.4.2 Gerresheimer AG
- 6.4.3 Schott AG
- 6.4.4 West Pharmaceutical Services Inc.
- 6.4.5 AptarGroup Inc.
- 6.4.6 Smurfit WestRock
- 6.4.7 Becton, Dickinson & Company
- 6.4.8 Catalent Inc.
- 6.4.9 CCL Industries Inc.
- 6.4.10 Klockner Pentaplast Group
- 6.4.11 Nipro Corporation
- 6.4.12 Vetter Pharma International GmbH
- 6.4.13 McKesson Corporation
- 6.4.14 FlexiTuff International Ltd.
- 6.4.15 W. L. Gore & Associates Inc.
- 6.4.16 Stevanato Group
- 6.4.17 Corning Incorporated
- 6.4.18 Owen-Illinois Inc.
- 6.4.19 SGD Pharma
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-space and Unmet-need Assessment







