 |
市場調查報告書
商品編碼
1889163
全球一次性內視鏡市場按類型、臨床用途、應用、最終用戶和地區分類-預測至2030年Disposable Endoscopes Market by Type (GI Endoscopes, Urology Endoscopes, Laryngoscopes), Clinical Usage (Diagnostic, Surgical), Application (Urology, Gastroenterology, ENT Endoscopy), End User (Hospitals, ASCs, Clinics), Region - Global Forecast to 2030 |
||||||
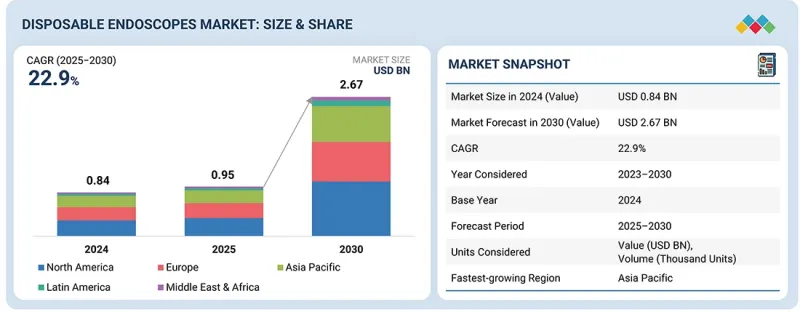
全球一次性內視鏡市場預計將從 2025 年的 9.5 億美元成長到 2030 年的 26.7 億美元,2025 年至 2030 年的複合年成長率為 22.9%。
| 調查範圍 | |
|---|---|
| 調查期 | 2024-2033 |
| 基準年 | 2024 |
| 預測期 | 2025-2030 |
| 單元 | 10億美元 |
| 部分 | 類型、臨床用途、應用、最終用戶、地區 |
| 目標區域 | 北美、歐洲、亞太地區、拉丁美洲、中東和非洲 |
由於慢性病盛行率上升、微創手術需求增加以及影像和光學技術的不斷進步,一次性內視鏡市場呈現強勁成長動能。新興市場蘊藏著巨大的機遇,因為醫療基礎設施的改善、手術量的增加以及醫療旅遊的蓬勃發展推動了先進內視鏡解決方案的普及。然而,感染風險、消毒和再處理流程不完善以及手術費用高昂等挑戰可能會阻礙市場成長並限制其應用,尤其是在對成本敏感且資源有限的醫療環境中。
預計到 2024 年,支氣管鏡細分市場將在一次性內視鏡市場中實現第二高的成長率。
依類型分類,一次性內視鏡市場可分為泌尿系統內視鏡、支氣管鏡、膀胱鏡、喉鏡、胃腸內視鏡、關節鏡和其他一次性內視鏡。其中,支氣管鏡市場預計將在2024年佔據第二大市場佔有率,這主要得益於全球呼吸系統疾病(尤其是肺癌)發病率的不斷上升。根據美國癌症協會預測,2025年美國將新增約226,650例肺癌病例,並有124,730例相關死亡病例。同時,世界癌症研究基金會報告稱,2022年全球新增肺癌病例達2,480,675例。日益加重的疾病負擔顯著推動了對支氣管鏡檢查的需求,使其能夠用於早期診斷和治療。一次性支氣管鏡因其有助於防止交叉感染、淘汰重複處理以及確保在急診和加護治療環境中性能穩定可靠,而日益受到青睞。此外,成像技術的進步、易用性以及醫院和門診診所的日益普及,進一步推動了這一領域的成長,使支氣管鏡成為一次性內視鏡市場的第二大促進因素。
2024年,外科手術領域在一次性內視鏡市場中佔據最大佔有率。
根據臨床用途,一次性內視鏡市場可分為診斷和手術兩大類。 2024年,手術類內視鏡佔據了最大的市場。這一成長主要得益於微創手術在包括胃腸病學、泌尿系統、呼吸系統醫療設備和婦科在內的多個專科領域的日益普及。外科醫生之所以青睞一次性內視鏡,是因為它們具有卓越的無菌性、可靠的性能,並且消除了交叉感染和再處理錯誤帶來的風險。隨著癌症、腎臟病和呼吸系統疾病等慢性病盛行率的不斷上升,需要精準可視化和可靠器械操作的外科手術也日益增多。此外,高解析度成像和符合人體工學設計的先進一次性內視鏡的廣泛應用,正在提高手術效率和安全性。醫院和門診手術中心正擴大轉向使用一次性器械,以加強感染控制和提高運作效率。這些因素使得手術類內視鏡成為一次性內視鏡市場的主要驅動力。
預計在預測期內,歐洲將佔據一次性內視鏡市場第二大佔有率。
全球一次性內視鏡市場分為五個地區:北美、歐洲、亞太、拉丁美洲以及中東和非洲。受人口結構變化、高發病率和先進的醫療保健體係等因素的共同推動,歐洲在2024年佔據了第二大市場佔有率。該地區正經歷顯著的人口老化,預計到2050年,德國65歲以上人口將增加41%,達到2,400萬人。此外,到2022年,英國19%的人口將達到或超過65歲,這將推動對診斷檢測和微創手術的需求成長。慢性病和癌症的增加也推動了對一次性內視鏡的需求,據報道,到2024年,53.7%的德國成年人患有慢性病,而英國(2022年)和德國(2023年)的癌症相關死亡人數分別達到138,579人和230,292人。這些因素,加上一流的醫院、先進醫療技術的採用以及支持性的法規結構,創造了強大的市場環境,使歐洲成為全球一次性內視鏡市場的關鍵成長區域。
本報告分析了全球一次性內視鏡市場,提供了關鍵促進因素和限制因素、競爭格局和未來趨勢的資訊。
目錄
第1章 引言
第2章執行摘要
第3章 主要發現
- 一次性內視鏡市場概覽
- 北美一次性內視鏡市場(以最終用戶分類)
- 一次性內視鏡市場地域概覽(2024 年)
- 地理細分:一次性內視鏡市場規模(2025-2030 年)
- 一次性內視鏡市場:發展中市場與已開發市場對比
第4章 市場概覽
- 市場動態
- 促進要素
- 抑制因素
- 機會
- 任務
- 未滿足的需求和閒置頻段
- 一次性內視鏡市場尚未滿足的需求
- 閒置頻段機會
- 相互關聯的市場與跨產業機遇
- 互聯市場
- 跨職能機會
- 一級/二級/三級公司的策略性舉措
第5章 產業趨勢
- 波特五力分析
- 宏觀經濟展望
- GDP趨勢與預測
- 內視鏡設備產業的全球趨勢
- 全球軟性內視鏡產業趨勢
- 供應鏈分析
- 價值鏈分析
- 生態系分析
- 定價分析
- 主要企業一次性內視鏡平均售價趨勢(2022-2024)
- 一次性內視鏡平均售價趨勢(按類型分類)(2022-2024 年)
- 2022-2024年各地區一次內視鏡平均售價趨勢
- 貿易分析
- 進口場景(HS編碼9018)
- 出口資料(HS編碼9018)
- 重大會議和活動(2025-2027)
- 影響客戶業務的趨勢/顛覆性因素
- 投資和資金籌措方案
- 案例研究分析
- 2025年美國關稅對一次性內視鏡市場的影響
- 主要關稅稅率
- 價格影響分析
- 對國家的影響
- 對終端用戶產業的影響
第6章:技術進步、人工智慧影響、專利、創新與未來應用
- 主要新技術
- CMOS/CCD成像感測器
- 光纖照明系統
- 互補技術
- 技術/產品藍圖
- 短期 | 基礎建設與早期商業化(2025-2027 年)
- 中期規劃 | 擴張與標準化(2027-2030 年)
- 長期 |大規模商業化與顛覆性變革(2030-2035 年及以後)
- 專利分析
- 未來應用
- 基於人工智慧的可視化和診斷支持
- 可生物分解且環保的內視鏡
- 內建感應器的智慧型內視鏡
- 人工智慧/生成式人工智慧對一次內視鏡市場的影響
- 一次內視鏡處理的最佳實踐
- 人工智慧在一次性內視鏡市場應用案例研究
- 相互關聯的相鄰生態系及其對市場參與者的影響
- 一次性內視鏡市場客戶對生成式人工智慧技術的接受程度
第7章永續性與監管環境
- 地方法規和合規性
- 監管機構、政府機構和其他組織
- 業界標準
- 對永續性的承諾
- 永續性影響和監管政策舉措
- 認證、標籤和環境標準
第8章:顧客狀況與購買行為
- 決策流程
- 買方相關利益者和採購評估標準
- 採購過程中的關鍵相關利益者
- 主要採購標準
- 招募障礙和內部挑戰
- 來自各個終端使用者產業的未滿足需求
- 市場盈利
- 潛在收入
- 成本動態
- 關鍵應用領域的利潤機會
第9章:一次性內視鏡市場(按類型分類)
- 泌尿系統視鏡檢查
- 支氣管鏡檢查
- 膀胱鏡
- 胃腸內視鏡檢查
- 喉鏡
- 關節鏡
- 其他一次內視鏡
第10章:依臨床用途分類的一次性內視鏡市場
- 外科用途
- 診斷用途
第11章:一次性內視鏡市場(按應用領域分類)
- 泌尿系統
- 支氣管鏡檢查
- 胃腸病學
- 耳鼻喉科
- 其他用途
第12章:以最終用戶分類的一次性內視鏡市場
- 醫院
- 門診手術中心
- 診所
- 其他最終用戶
第13章:按地區分類的一次性內視鏡市場
- 北美洲
- 北美宏觀經濟展望
- 美國
- 加拿大
- 歐洲
- 歐洲宏觀經濟展望
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 其他歐洲
- 亞太地區
- 亞太宏觀經濟展望
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 其他亞太地區
- 拉丁美洲
- 拉丁美洲宏觀經濟展望
- 巴西
- 墨西哥
- 其他拉丁美洲
- 中東和非洲
- 中東和非洲宏觀經濟展望
- 海灣合作理事會國家
- 其他中東和非洲地區
第14章 競爭格局
- 主要參與企業的策略/優勢
- 收入分析(2020-2024)
- 市佔率分析(2024 年)
- 企業評估矩陣:主要企業(2024)
- 公司評估矩陣:Start-Ups/中小企業(2024 年)
- 公司估值和財務指標
- 品牌/產品對比
- 競爭場景
第15章:公司簡介
- 主要企業
- AMBU A/S
- BOSTON SCIENTIFIC CORPORATION
- OLYMPUS CORPORATION
- HOYA CORPORATION
- VERATHON INC.
- KARL STORZ SE & CO. KG
- HUNAN VATHIN MEDICAL INSTRUMENT CO., LTD.
- INNOVEX MEDICAL CO., LTD.
- SCIVITA MEDICAL
- GUANGZHOU RED PINE MEDICAL INSTRUMENT CO., LTD.
- 其他公司
- THE COOPER COMPANIES, INC.
- ZHUHAI PUSEN MEDICAL TECHNOLOGY CO., LTD.
- UROVIU CORPORATION
- OTU MEDICAL
- GI VIEW LTD.
- TSC LIFE
- RICHARD WOLF GMBH
- DAICHUAN MEDICA
- NEOSCOPE INC.
- INTEGRATED ENDOSCOPY
- HUGER MEDICAL INSTRUMENT CO., LTD.
- XENOCOR
- MACROLUX MEDICAL TECHNOLOGY CO., LTD.
- SHENZHEN HUGEMED MEDICAL TECHNICAL DEVELOPMENT CO., LTD.
- ZHEJIANG GEYI MEDICAL DEVICES CO., LTD.
第16章調查方法
第17章附錄
The global disposable endoscopes market is projected to reach USD 2.67 billion by 2030, growing from USD 0.95 billion in 2025, at a CAGR of 22.9% from 2025 to 2030.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2033 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Type, Clinical Usage, Application, End User, and Region |
| Regions covered | North America, Europe, APAC, LATAM, MEA |
The disposable endoscopes market is experiencing strong growth driven by the rising prevalence of chronic diseases, increasing demand for minimally invasive procedures, and ongoing advancements in imaging and optical technologies. Emerging markets offer substantial opportunities due to improving healthcare infrastructure, expanding procedure volumes, and the growth of medical tourism, which facilitate the wider adoption of advanced endoscopic solutions. However, market growth faces challenges such as infection risks, insufficient sterilization and reprocessing practices, and high procedural costs, which may limit broader adoption-particularly in cost-sensitive and resource-limited healthcare settings.

The bronchoscopes segment accounted for the second-largest growth in the disposable endoscopes market in 2024.
By type, the disposable endoscopes market is divided into urology endoscopes, bronchoscopes, cystoscopes, laryngoscopes, GI endoscopes, arthroscopes, and other disposable endoscopes. Among these, the bronchoscopes segment held the second-largest market share in 2024, driven by the increasing global incidence of respiratory diseases, especially lung cancer. According to the American Cancer Society, in 2025, the US is expected to see about 226,650 new lung cancer cases and 124,730 related deaths, while the World Cancer Research Fund reported 2,480,675 new cases worldwide in 2022. This rising disease burden has significantly boosted the demand for bronchoscopy procedures for early diagnosis and treatment. Disposable bronchoscopes are increasingly chosen because they help prevent cross-contamination, eliminate the need for reprocessing, and ensure consistent performance in emergency and intensive care situations. Additionally, advancements in imaging technology and maneuverability, along with growing adoption by hospitals and outpatient clinics, are further driving the growth of this segment, making bronchoscopes the second largest contributor to the disposable endoscopes market.
The surgical segment accounted for the largest share of the disposable endoscopes market in 2024.
By clinical practice, the disposable endoscopes market is divided into diagnostic and surgical uses. In 2024, the surgical use segment held the largest market share. This growth is driven by the rising adoption of minimally invasive surgical procedures across various specialties, including gastroenterology, urology, pulmonology, and gynecology. Surgeons favor disposable endoscopes for their superior sterility, reliable performance, and ability to eliminate risks associated with cross-contamination and reprocessing errors. The increasing prevalence of chronic conditions such as cancer, kidney disorders, and respiratory diseases has led to a rise in surgical procedures that require precise visualization and dependable instrumentation. Additionally, the availability of advanced single-use endoscopes with high-definition imaging and ergonomic designs improves procedural efficiency and safety. Hospitals and outpatient surgical centers are increasingly shifting toward single-use devices to enhance infection control and streamline operations. Collectively, these factors establish the surgical usage segment as the leading contributor to the disposable endoscopes market by clinical application.
Europe accounted for the second-largest position in the disposable endoscopes market during the forecast period.
The global disposable endoscopes market is divided into five major regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2024, Europe held the second-largest share in this market. This is due to a mix of demographic trends, high disease rates, and advanced healthcare systems. The region is experiencing notable population aging, with Germany's population aged 65 and older projected to increase by 41% to 24 million by 2050. Additionally, 19% of the UK population was already over 65 in 2022, leading to higher demand for diagnostic and minimally invasive procedures. The growing burden of chronic diseases and cancer also drives the need for disposable endoscopes, with 53.7% of adults in Germany reporting chronic conditions in 2024, and cancer-related deaths reaching 138,579 in England (2022) and 230,292 in Germany (2023). Coupled with well-established hospitals, the adoption of advanced medical technology, and supportive regulatory frameworks, these factors together create a strong market environment, making Europe a key growth region in the global disposable endoscopes market.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1 -40%, Tier 2 -30%, and Tier 3 -30%
- By Designation: C-level -50%, Director level -30%, and Others -20%
- By Region: North America -30%, Europe - 25%, Asia Pacific -20%, Latin America -15%, and Middle East & Africa -10%
Notes:
Companies are classified into tiers based on their total revenue. The tiers are as follows: Tier 1 = > USD 10.0 billion, tier 2 = USD 1.0 billion to USD 10.0 billion, and tier 3 = < USD 1.0 billion
C-level primaries include CEOs, CFOs, COOs, and VPs.
Others include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
The players operating in the disposable endoscopes market include Olympus Corporation (Japan), Boston Scientific Corporation (US), HOYA Corporation (Japan), Ambu A/s (Denmark), Karl Storz SE & Co. KG (Germany), Hunan Vathin Medical Instrument Co., Ltd. (China), Zhuhai Pusen Medical Technology Co., Ltd. (China), GI View Ltd (Isareal), Richard Wolf GmbH (Germany), Daichuan medica (China), Scivita Medical (China), Uroviu Corporation (US), Neoscope Inc. (US), Verathon Inc. A Ropper Technologies Company (US), Integrated Endoscopy (US), HUGER Medical Instrument Co., Ltd. (China), Guangzhou Red Pine Medical Instrument Co. Ltd. (China), Nanchang WOEK Medical Technology Co., Ltd (China), Innovex Medical Co., Ltd. (China), Xenocor (US), MacroLux Medical Technology Co., Ltd. (China), THE COOPER COMPANIES, INC. (US), Shenzhen HugeMed Medical Technical Development Co., Ltd. (China), and Xi'an Haiye Medical Equipment Co., Ltd. (China).
Research Coverage
This report examines the disposable endoscopes market based on type, application, clinical use, end user, and region. It also explores factors such as drivers, restraints, opportunities, and challenges influencing market growth, and provides an overview of the competitive landscape for market leaders. Additionally, the report analyzes micro markets concerning their individual growth trends and forecasts the revenue of market segments across five major regions and their respective countries.
Reasons to Buy the Report
The report will help both established and smaller firms understand the market, which, in turn, will assist them in increasing their market share. Firms purchasing the report can use one or a combination of the strategies mentioned below to strengthen their position in the market presence.
This report provides insights into the following pointers:
- Analysis of key drivers (Increasing investments, funds, and grants by government and other organizations, growing focus of hospitals to expand endoscopic units, increasing preference for minimally invasive surgeries, rising need for endoscopy to diagnose & treat target diseases), restraints (High overhead costs of endoscopy procedures), opportunities (Rapidly developing healthcare sector in emerging countries), challenges (Environmental concerns related to medical waste disposal, limited reimbursement policies)
- Market Penetration: Complete knowledge of the spectrum of products presented by the major companies in the disposable endoscopes market
- Product Development/Innovation: Comprehensive understanding of the forthcoming trends, research and development initiatives, and product launches within the disposable endoscopes market
- Market Development: Complete knowledge about profitable developing regions
- Market Diversification: Exhaustive knowledge of new goods, expanding geographies, and current changes in the disposable endoscopes industry help to diversify the market
- Competitive Assessment: In-depth assessment of market share, growth strategies and product offerings of leading players like Olympus Corporation (Japan), Boston Scientific Corporation (US), HOYA Corporation (Japan), Ambu A/s (Denmark), Karl Storz SE & Co. KG (Germany), Hunan Vathin Medical Instrument Co., Ltd. (China), Zhuhai Pusen Medical Technology Co., Ltd. (China), and among others.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKETS COVERED & REGIONAL SEGMENTATION
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
2 EXECUTIVE SUMMARY
- 2.1 KEY INSIGHTS & MARKET HIGHLIGHTS
- 2.2 KEY MARKET PARTICIPANTS: INSIGHTS & DEVELOPMENTS
- 2.3 DISRUPTIVE TRENDS SHAPING MARKET GROWTH
- 2.4 HIGH-GROWTH SEGMENTS & EMERGING FRONTIERS
- 2.5 SNAPSHOT: GLOBAL MARKET SIZE, GROWTH RATE, AND FORECAST
3 PREMIUM INSIGHTS
- 3.1 DISPOSABLE ENDOSCOPES MARKET OVERVIEW
- 3.2 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY END USER
- 3.3 GEOGRAPHIC SNAPSHOT OF DISPOSABLE ENDOSCOPES MARKET (2024)
- 3.4 GEOGRAPHIC MIX: DISPOSABLE ENDOSCOPES MARKET, 2025-2030 (USD MILLION)
- 3.5 DISPOSABLE ENDOSCOPES MARKET: DEVELOPING VS. DEVELOPED MARKETS
4 MARKET OVERVIEW
- 4.1 INTRODUCTION
- 4.2 MARKET DYNAMICS
- 4.2.1 DRIVERS
- 4.2.1.1 Growing demand for infection control and regulatory push
- 4.2.1.2 Rising burden of chronic diseases
- 4.2.2 RESTRAINTS
- 4.2.2.1 Environmental impact and sustainability concerns
- 4.2.3 OPPORTUNITIES
- 4.2.3.1 Rapidly developing healthcare sector in emerging economies
- 4.2.4 CHALLENGES
- 4.2.4.1 High upfront expenditure per procedure
- 4.2.1 DRIVERS
- 4.3 UNMET NEEDS & WHITE SPACES
- 4.3.1 UNMET NEEDS IN DISPOSABLE ENDOSCOPES MARKET
- 4.3.2 WHITE SPACE OPPORTUNITIES
- 4.4 INTERCONNECTED MARKETS & CROSS-SECTOR OPPORTUNTIES
- 4.4.1 INTERCONNECTED MARKETS
- 4.4.2 CROSS-SECTOR OPPORTUNTIES
- 4.5 STRATEGIC MOVES BY TIER-1/2/3 PLAYERS
5 INDUSTRY TRENDS
- 5.1 PORTER'S FIVE FORCES ANALYSIS
- 5.1.1 THREAT OF NEW ENTRANTS
- 5.1.2 THREAT OF SUBSTITUTES
- 5.1.3 BARGAINING POWER OF BUYERS
- 5.1.4 BARGAINING POWER OF SUPPLIERS
- 5.1.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.2 MACROECONOMIC OUTLOOK
- 5.2.1 INTRODUCTION
- 5.2.2 GDP TRENDS AND FORECAST
- 5.2.3 TRENDS IN GLOBAL ENDOSCOPY EQUIPMENT IDUSTRY
- 5.2.4 TRENDS IN GLOBAL FLEXIBLE ENDOSCOPES INDUSTRY
- 5.3 SUPPLY CHAIN ANALYSIS
- 5.4 VALUE CHAIN ANALYSIS
- 5.5 ECOSYSTEM ANALYSIS
- 5.6 PRICING ANALYSIS
- 5.6.1 AVERAGE SELLING PRICE TREND OF DISPOSABLE ENDOSCOPES, BY KEY PLAYER (2022-2024)
- 5.6.2 AVERAGE SELLING PRICE TREND OF DISPOSABLE ENDOSCOPES, BY TYPE (2022-2024)
- 5.6.3 AVERAGE SELLING PRICE TREND OF DISPOSABLE ENDOSCOPES, BY REGION (2022-2024)
- 5.7 TRADE ANALYSIS
- 5.7.1 IMPORT SCENARIO (HS CODE 9018)
- 5.7.2 EXPORT DATA (HS CODE 9018)
- 5.8 KEY CONFERENCES & EVENTS, 2025-2027
- 5.9 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- 5.10 INVESTMENT & FUNDING SCENARIO
- 5.11 CASE STUDY ANALYSIS
- 5.11.1 CASE STUDY 1: ICU BRONCHOSCOPY PERFORMANCE AND ADOPTION
- 5.11.2 CASE STUDY 2: ERCP INFECTION-CONTROL CONVERSION PATHWAY
- 5.11.3 CASE STUDY 3: OPTIMIZING TRANSBRONCHIAL CRYOBIOPSY IN INTERSTITIAL LUNG DISEASE USING AMBU ASCOPE 5 BRONCHO HD
- 5.12 IMPACT OF 2025 US TARIFFS ON DISPOSABLE ENDOSCOPES MARKET
- 5.12.1 INTRODUCTION
- 5.12.2 KEY TARIFF RATES
- 5.12.3 PRICE IMPACT ANALYSIS
- 5.12.4 IMPACT ON COUNTRIES/REGIONS
- 5.12.4.1 North America
- 5.12.4.2 Europe
- 5.12.4.3 Asia Pacific
- 5.12.5 END-USE INDUSTRY IMPACT
6 TECHNOLOGICAL ADVANCEMENTS, AI-DRIVEN IMPACT, PATENTS, INNOVATIONS, AND FUTURE APPLICATIONS
- 6.1 KEY EMERGING TECHNOLOGIES
- 6.1.1 CMOS & CCD IMAGING SENSORS
- 6.1.2 FIBER-OPTIC ILLUMINATION SYSTEMS
- 6.2 COMPLEMENTARY TECHNOLOGIES
- 6.2.1 AI-ASSISTED IMAGE PROCESSING AND VISUALIZATION
- 6.3 TECHNOLOGY/PRODUCT ROADMAP
- 6.3.1 SHORT TERM (2025-2027) | FOUNDATION & EARLY COMMERCIALIZATION
- 6.3.2 MID TERM (2027-2030) | EXPANSION & STANDARDIZATION
- 6.3.3 LONG TERM (2030-2035+) | MASS COMMERCIALIZATION & DISRUPTION
- 6.4 PATENT ANALYSIS
- 6.4.1 INSIGHTS: JURISDICTION AND TOP APPLICANT ANALYSIS
- 6.5 FUTURE APPLICATIONS
- 6.5.1 AI-ENABLED VISUALIZATION AND DIAGNOSTIC SUPPORT
- 6.5.2 BIODEGRADABLE & ECO-FRIENDLY ENDOSCOPES
- 6.5.3 SMART ENDOSCOPES WITH EMBEDDED SENSORS
- 6.6 IMPACT OF AI/GEN AI ON DISPOSABLE ENDOSCOPES MARKET
- 6.6.1 TOP USE CASES & MARKET POTENTIAL
- 6.7 BEST PRACTICES IN DISPOSABLE ENDOSCOPES PROCESSING
- 6.7.1 CASE STUDIES OF AI IMPLEMENTATION IN DISPOSABLE ENDOSCOPES MARKET
- 6.7.2 INTERCONNECTED ADJACENT ECOSYSTEM & IMPACT ON MARKET PLAYERS
- 6.7.3 CLIENTS' READINESS TO ADOPT GENERATIVE AI IN DISPOSABLE ENDOSCOPES MARKET
7 SUSTAINABILITY & REGULATORY LANDSCAPE
- 7.1 REGIONAL REGULATIONS & COMPLIANCE
- 7.1.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 7.1.2 INDUSTRY STANDARDS
- 7.2 SUSTAINABILITY INITIATIVES
- 7.2.1 CARBON IMPACT AND ECO-APPLICATIONS OF DISPOSABLE ENDOSCOPES
- 7.2.1.1 Carbon impact reduction
- 7.2.1.2 Eco-applications
- 7.2.1 CARBON IMPACT AND ECO-APPLICATIONS OF DISPOSABLE ENDOSCOPES
- 7.3 SUSTAINABILITY IMPACT & REGULATORY POLICY INITIATIVES
- 7.4 CERTIFICATIONS, LABELING, AND ECO-STANDARDS
8 CUSTOMER LANDSCAPE & BUYER BEHAVIOR
- 8.1 DECISION-MAKING PROCESS
- 8.2 BUYER STAKEHOLDERS & BUYING EVALUATION CRITERIA
- 8.2.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 8.2.2 KEY BUYING CRITERIA
- 8.3 ADOPTION BARRIERS & INTERNAL CHALLENGES
- 8.4 UNMET NEEDS FROM VARIOUS END-USE INDUSTRIES
- 8.5 MARKET PROFITABILITY
- 8.5.1 REVENUE POTENTIAL
- 8.5.2 COST DYNAMICS
- 8.5.3 MARGIN OPPORTUNITIES IN KEY APPLICATIONS
9 DISPOSABLE ENDOSCOPES MARKET, BY TYPE
- 9.1 INTRODUCTION
- 9.2 UROLOGY ENDOSCOPES
- 9.2.1 UROLOGY ENDOSCOPES SEGMENT TO HOLD MAJOR SHARE OF MARKET
- 9.3 BRONCHOSCOPES
- 9.3.1 RISING PREVALENCE OF LUNG CANCER TO BOOST SEGMENTAL GROWTH
- 9.4 CYSTOSCOPES
- 9.4.1 GROWING BURDEN OF BLADDER CANCER TO AID SEGMENTAL GROWTH
- 9.5 GI ENDOSCOPES
- 9.5.1 STRINGENT REGULATORY RECOMMENDATIONS FROM US FDA TO ACCELERATE SHIFT TOWARD SINGLE-USE GI ENDOSCOPES
- 9.6 LARYNGOSCOPES
- 9.6.1 RISING INFECTION CONTROL AND INCREASING LARYNGEAL DISORDER BURDEN TO DRIVE SEGMENTAL GROWTH
- 9.7 ARTHROSCOPES
- 9.7.1 RISING GERIATRIC POPULATION AND GROWING INCIDENCE OF ORTHOPEDIC DISEASES TO BOOST MARKET GROWTH
- 9.8 OTHER DISPOSABLE ENDOSCOPES
10 DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE
- 10.1 INTRODUCTION
- 10.2 SURGICAL USAGE
- 10.2.1 RISING ADOPTION OF MINIMALLY INVASIVE SURGERIES TO DRIVE SEGMENTAL GROWTH
- 10.3 DIAGNOSTIC USAGE
- 10.3.1 RISING PREFERENCE FOR SAFE AND EFFICIENT DIAGNOSTIC PROCEDURES TO DRIVE ADOPTION OF DISPOSABLE ENDOSCOPES
11 DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION
- 11.1 INTRODUCTION
- 11.2 UROLOGY
- 11.2.1 RISING PREVALENCE OF URINARY TRACT DISEASES AND CANCERS TO AID MARKET GROWTH
- 11.3 BRONCHOSCOPY
- 11.3.1 RISING INFECTION CONTROL NEEDS TO DRIVE GROWTH OF BRONCHOSCOPY SEGMENT
- 11.4 GASTROENTEROLOGY
- 11.4.1 TECHNOLOGICAL ADVANCEMENTS AND GROWING PROCEDURAL VOLUMES IN HOSPITAL ENDOSCOPY UNITS TO FUEL DEMAND
- 11.5 ENT
- 11.5.1 ABILITY OF DISPOSABLE ENT ENDOSCOPES TO REDUCE CROSS-CONTAMINATION RISKS TO DRIVE DEMAND
- 11.6 OTHER APPLICATIONS
12 DISPOSABLE ENDOSCOPES MARKET, BY END USER
- 12.1 INTRODUCTION
- 12.2 HOSPITALS
- 12.2.1 HOSPITALS TO DOMINATE DISPOSABLE ENDOSCOPES MARKET DURING FORECAST PERIOD
- 12.3 AMBULATORY SURGERY CENTERS
- 12.3.1 GROWING PREFERENCE FOR OUTPATIENT SURGERIES AND TECHNOLOGICAL ADVANCEMENTS TO ACCELERATE GROWTH
- 12.4 CLINICS
- 12.4.1 RISING ADOPTION OF OUTPATIENT DIAGNOSTICS AND MINOR PROCEDURES TO DRIVE SEGMENTAL GROWTH
- 12.5 OTHER END USERS
13 DISPOSABLE ENDOSCOPES MARKET, BY REGION
- 13.1 INTRODUCTION
- 13.2 NORTH AMERICA
- 13.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 13.2.2 US
- 13.2.2.1 US to dominate North American disposable endoscopes market during forecast period
- 13.2.3 CANADA
- 13.2.3.1 Stringent infection control standards and rising chronic disease burden to adoption of disposable endoscopes
- 13.3 EUROPE
- 13.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 13.3.2 GERMANY
- 13.3.2.1 Germany to dominate European disposable endoscopes market
- 13.3.3 UK
- 13.3.3.1 Growing aging population and infection control focus to boost market
- 13.3.4 FRANCE
- 13.3.4.1 Increase in health expenditure and high prevalence of cancer to stimulate market growth
- 13.3.5 ITALY
- 13.3.5.1 Increasing aging population and prevalence of chronic diseases to boost adoption of disposable endoscopes
- 13.3.6 SPAIN
- 13.3.6.1 Rising aging population to drive market growth
- 13.3.7 REST OF EUROPE
- 13.4 ASIA PACIFIC
- 13.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 13.4.2 CHINA
- 13.4.2.1 China to account for largest share of APAC region in disposable endoscopes market
- 13.4.3 JAPAN
- 13.4.3.1 Increasing chronic diseases and geriatric population to drive Japan's growth in market
- 13.4.4 INDIA
- 13.4.4.1 Rising disease burden and aging population to position India as key growth hub in Asia Pacific
- 13.4.5 AUSTRALIA
- 13.4.5.1 Rising cancer burden and expanding screening programs to support disposable endoscopes adoption in Australia
- 13.4.6 SOUTH KOREA
- 13.4.6.1 Robust cancer screening programs to drive strong adoption of disposable endoscopes
- 13.4.7 REST OF ASIA PACIFIC
- 13.5 LATIN AMERICA
- 13.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 13.5.2 BRAZIL
- 13.5.2.1 Brazil to account for largest share of LATAM market in 2024
- 13.5.3 MEXICO
- 13.5.3.1 Expanding healthcare infrastructure to strengthen Mexico's position in global market
- 13.5.4 REST OF LATIN AMERICA
- 13.6 MIDDLE EAST & AFRICA
- 13.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
- 13.6.2 GCC COUNTRIES
- 13.6.2.1 Increasing healthcare investments and hospital expansions to drive market growth
- 13.6.3 REST OF MIDDLE EAST & AFRICA
14 COMPETITIVE LANDSCAPE
- 14.1 INTRODUCTION
- 14.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 14.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN DISPOSABLE ENDOSCOPES MARKET
- 14.3 REVENUE ANALYSIS, 2020-2024
- 14.4 MARKET SHARE ANALYSIS, 2024
- 14.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 14.5.1 STARS
- 14.5.2 EMERGING LEADERS
- 14.5.3 PERVASIVE PLAYERS
- 14.5.4 PARTICIPANTS
- 14.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 14.5.5.1 Company footprint
- 14.5.5.2 Region footprint
- 14.5.5.3 Type footprint
- 14.5.5.4 Application footprint
- 14.5.5.5 End-user footprint
- 14.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 14.6.1 PROGRESSIVE COMPANIES
- 14.6.2 RESPONSIVE COMPANIES
- 14.6.3 DYNAMIC COMPANIES
- 14.6.4 STARTING BLOCKS
- 14.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 14.6.5.1 Detailed list of key startups/SMEs
- 14.6.5.2 Competitive benchmarking of key startups/SMEs
- 14.7 COMPANY VALUATION & FINANCIAL METRICS
- 14.7.1 FINANCIAL METRICS
- 14.7.2 COMPANY VALUATION
- 14.8 BRAND/PRODUCT COMPARISON
- 14.9 COMPETITIVE SCENARIO
- 14.9.1 PRODUCT LAUNCHES & APPROVALS
- 14.9.2 DEALS
- 14.9.3 EXPANSIONS
15 COMPANY PROFILES
- 15.1 KEY PLAYERS
- 15.1.1 AMBU A/S
- 15.1.1.1 Business overview
- 15.1.1.2 Products offered
- 15.1.1.3 Recent developments
- 15.1.1.3.1 Product launches & approvals
- 15.1.1.3.2 Expansions
- 15.1.1.4 MnM view
- 15.1.1.4.1 Right to win
- 15.1.1.4.2 Strategic choices
- 15.1.1.4.3 Weaknesses & competitive threats
- 15.1.2 BOSTON SCIENTIFIC CORPORATION
- 15.1.2.1 Business overview
- 15.1.2.2 Products offered
- 15.1.2.3 Recent developments
- 15.1.2.3.1 Product approvals
- 15.1.2.3.2 Deals
- 15.1.2.4 MnM view
- 15.1.2.4.1 Right to win
- 15.1.2.4.2 Strategic choices
- 15.1.2.4.3 Weaknesses & competitive threats
- 15.1.3 OLYMPUS CORPORATION
- 15.1.3.1 Business overview
- 15.1.3.2 Products offered
- 15.1.3.3 Recent developments
- 15.1.3.3.1 Product approvals
- 15.1.3.3.2 Deals
- 15.1.3.4 MnM view
- 15.1.3.4.1 Right to win
- 15.1.3.4.2 Strategic choices
- 15.1.3.4.3 Weaknesses & competitive threats
- 15.1.4 HOYA CORPORATION
- 15.1.4.1 Business overview
- 15.1.4.2 Products offered
- 15.1.4.3 Recent developments
- 15.1.4.3.1 Deals
- 15.1.4.4 MnM view
- 15.1.4.4.1 Right to win
- 15.1.4.4.2 Strategic choices
- 15.1.4.4.3 Weaknesses & competitive threats
- 15.1.5 VERATHON INC.
- 15.1.5.1 Business overview
- 15.1.5.2 Products offered
- 15.1.5.3 Recent developments
- 15.1.5.3.1 Product launches
- 15.1.5.4 MnM view
- 15.1.5.4.1 Right to win
- 15.1.5.4.2 Strategic choices
- 15.1.5.4.3 Weaknesses & competitive threats
- 15.1.6 KARL STORZ SE & CO. KG
- 15.1.6.1 Business overview
- 15.1.6.2 Products offered
- 15.1.6.3 Recent developments
- 15.1.6.3.1 Product launches
- 15.1.6.3.2 Deals
- 15.1.7 HUNAN VATHIN MEDICAL INSTRUMENT CO., LTD.
- 15.1.7.1 Business overview
- 15.1.7.2 Products offered
- 15.1.7.3 Recent developments
- 15.1.7.3.1 Deals
- 15.1.8 INNOVEX MEDICAL CO., LTD.
- 15.1.8.1 Business overview
- 15.1.8.2 Products offered
- 15.1.9 SCIVITA MEDICAL
- 15.1.9.1 Business overview
- 15.1.9.2 Products offered
- 15.1.10 GUANGZHOU RED PINE MEDICAL INSTRUMENT CO., LTD.
- 15.1.10.1 Business overview
- 15.1.10.2 Products offered
- 15.1.1 AMBU A/S
- 15.2 OTHER PLAYERS
- 15.2.1 THE COOPER COMPANIES, INC.
- 15.2.2 ZHUHAI PUSEN MEDICAL TECHNOLOGY CO., LTD.
- 15.2.3 UROVIU CORPORATION
- 15.2.4 OTU MEDICAL
- 15.2.5 GI VIEW LTD.
- 15.2.6 TSC LIFE
- 15.2.7 RICHARD WOLF GMBH
- 15.2.8 DAICHUAN MEDICA
- 15.2.9 NEOSCOPE INC.
- 15.2.10 INTEGRATED ENDOSCOPY
- 15.2.11 HUGER MEDICAL INSTRUMENT CO., LTD.
- 15.2.12 XENOCOR
- 15.2.13 MACROLUX MEDICAL TECHNOLOGY CO., LTD.
- 15.2.14 SHENZHEN HUGEMED MEDICAL TECHNICAL DEVELOPMENT CO., LTD.
- 15.2.15 ZHEJIANG GEYI MEDICAL DEVICES CO., LTD.
16 RESEARCH METHODOLOGY
- 16.1 RESEARCH DATA
- 16.1.1 SECONDARY DATA
- 16.1.1.1 Key data from secondary sources
- 16.1.2 PRIMARY DATA
- 16.1.2.1 Key data from primary sources
- 16.1.2.2 Key industry insights
- 16.1.1 SECONDARY DATA
- 16.2 MARKET SIZE ESTIMATION
- 16.3 MARKET BREAKDOWN & DATA TRIANGULATION
- 16.4 MARKET RANKING ANALYSIS
- 16.5 STUDY ASSUMPTIONS
- 16.6 RESEARCH LIMITATIONS
- 16.6.1 METHODOLOGY-RELATED LIMITATIONS
- 16.6.2 SCOPE-RELATED LIMITATIONS
- 16.7 RISK ASSESSMENT
17 APPENDIX
- 17.1 DISCUSSION GUIDE
- 17.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 17.3 CUSTOMIZATION OPTIONS
- 17.4 RELATED REPORTS
- 17.5 AUTHOR DETAILS
List of Tables
- TABLE 1 DISPOSABLE ENDOSCOPES MARKET: INCLUSIONS & EXCLUSIONS
- TABLE 2 STANDARD CURRENCY CONVERSION RATES
- TABLE 3 DISPOSABLE ENDOSCOPES MARKET: PORTER'S FIVE FORCES ANALYSIS
- TABLE 4 GDP PERCENTAGE CHANGE, BY KEY COUNTRY, 2021-2030
- TABLE 5 AVERAGE SELLING PRICE TREND OF DISPOSABLE ENDOSCOPES, BY KEY PLAYER, 2022-2024 (USD)
- TABLE 6 AVERAGE SELLING PRICE TREND OF DISPOSABLE ENDOSCOPES, BY TYPE, 2022-2024 (USD)
- TABLE 7 AVERAGE SELLING PRICE TREND OF DISPOSABLE ENDOSCOPES, BY REGION, 2022-2024 (USD)
- TABLE 8 IMPORT DATA FOR DISPOSABLE ENDOSCOPE PRODUCTS UNDER HS CODE 9018, BY COUNTRY, 2019-2024 (USD THOUSAND)
- TABLE 9 EXPORT DATA FOR DISPOSABLE ENDOSCOPE PRODUCTS UNDER HS CODE 9018, BY COUNTRY, 2019-2024 (USD THOUSAND)
- TABLE 10 DISPOSABLE ENDOSCOPES MARKET: DETAILED LIST OF KEY CONFERENCES & EVENTS (2025-2027)
- TABLE 11 US-ADJUSTED RECIPROCAL TARIFF RATES
- TABLE 12 KEY PRODUCT-RELATED TARIFFS EFFECTIVE FOR ORTHODONTIC SUPPLIES
- TABLE 13 DISPOSABLE ENDOSCOPES MARKET: LIST OF PATENTS, 2022-2025
- TABLE 14 TOP USE CASES & MARKET POTENTIAL
- TABLE 15 DISPOSABLE ENDOSCOPES MARKET: CASE STUDIES RELATED TO AI IMPLEMENTATION
- TABLE 16 INTERCONNECTED ADJACENT ECOSYSTEM & IMPACT ON MARKET PLAYERS
- TABLE 17 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 18 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 19 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 20 LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 21 MIDDLE EAST & AFRICA AND GCC COUNTRIES: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 22 GLOBAL STANDARDS IN DISPOSABLE ENDOSCOPES MARKET
- TABLE 23 CERTIFICATIONS, LABELING, AND ECO-STANDARDS IN DISPOSABLE ENDOSCOPES MARKET
- TABLE 24 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR DISPOSABLE ENDOSCOPES
- TABLE 25 KEY BUYING CRITERIA FOR TOP THREE DISPOSABLE ENDOSCOPE TYPES
- TABLE 26 DISPOSABLE ENDOSCOPES MARKET: UNMET NEEDS/END-USER EXPECTATIONS
- TABLE 27 DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 28 UROLOGY ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 29 BRONCHOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 30 CYSTOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 31 GI ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 32 LARYNGOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 33 ARTHROSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 34 OTHER DISPOSABLE ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 35 DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 36 DISPOSABLE ENDOSCOPES MARKET FOR SURGICAL USAGE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 37 DISPOSABLE ENDOSCOPES MARKET FOR DIAGNOSTIC USAGE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 38 DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 39 DISPOSABLE ENDOSCOPES MARKET FOR UROLOGY APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 40 DISPOSABLE ENDOSCOPES MARKET FOR BRONCHOSCOPY APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 41 DISPOSABLE ENDOSCOPES MARKET FOR GASTROENTEROLOGY APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 42 DISPOSABLE ENDOSCOPES MARKET FOR ENT APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 43 DISPOSABLE ENDOSCOPES MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 44 DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 45 DISPOSABLE ENDOSCOPES MARKET FOR HOSPITALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 46 DISPOSABLE ENDOSCOPES MARKET FOR AMBULATORY SURGERY CENTERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 47 DISPOSABLE ENDOSCOPES MARKET FOR CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 48 DISPOSABLE ENDOSCOPES MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 49 DISPOSABLE ENDOSCOPES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 50 DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (THOUSAND UNITS)
- TABLE 51 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 52 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 53 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (THOUSAND UNITS)
- TABLE 54 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 55 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 56 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 57 US: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 58 US: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 59 US: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 60 US: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 61 CANADA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 62 CANADA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 63 CANADA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 64 CANADA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 65 EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 66 EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 67 EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (THOUSAND UNITS)
- TABLE 68 EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 69 EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 70 EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 71 GERMANY: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 72 GERMANY: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 73 GERMANY: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 74 GERMANY: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 75 UK: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 76 UK: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 77 UK: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 78 UK: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 79 FRANCE: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 80 FRANCE: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 81 FRANCE: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 82 FRANCE: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 83 ITALY: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 84 ITALY: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 85 ITALY: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 86 ITALY: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 87 SPAIN: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 88 SPAIN: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 89 SPAIN: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 90 SPAIN: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 91 REST OF EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 92 REST OF EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 93 REST OF EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 94 REST OF EUROPE: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 95 ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 96 ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 97 ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (THOUSAND UNITS)
- TABLE 98 ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 99 ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 100 ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 101 CHINA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 102 CHINA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 103 CHINA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 104 CHINA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 105 JAPAN: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 106 JAPAN: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 107 JAPAN: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 108 JAPAN: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 109 INDIA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 110 INDIA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 111 INDIA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 112 INDIA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 113 AUSTRALIA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 114 AUSTRALIA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 115 AUSTRALIA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 116 AUSTRALIA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 117 SOUTH KOREA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 118 SOUTH KOREA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 119 SOUTH KOREA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 120 SOUTH KOREA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 121 REST OF ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 122 REST OF ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 123 REST OF ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 124 REST OF ASIA PACIFIC: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 125 LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 126 LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 127 LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (THOUSAND UNITS)
- TABLE 128 LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 129 LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 130 LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 131 BRAZIL: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 132 BRAZIL: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 133 BRAZIL: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 134 BRAZIL: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 135 MEXICO: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 136 MEXICO: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 137 MEXICO: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 138 MEXICO: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 139 REST OF LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 140 REST OF LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 141 REST OF LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 142 REST OF LATIN AMERICA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 143 MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 144 MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 145 MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (THOUSAND UNITS)
- TABLE 146 MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 147 MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 148 MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 149 GCC COUNTRIES: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 150 GCC COUNTRIES: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 151 GCC COUNTRIES: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 152 GCC COUNTRIES: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 153 REST OF MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 154 REST OF MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY CLINICAL USAGE, 2023-2030 (USD MILLION)
- TABLE 155 REST OF MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 156 REST OF MIDDLE EAST & AFRICA: DISPOSABLE ENDOSCOPES MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 157 OVERVIEW OF STRATEGIES DEPLOYED BY KEY PLAYERS IN DISPOSABLE ENDOSCOPES MARKET
- TABLE 158 DISPOSABLE ENDOSCOPES MARKET: DEGREE OF COMPETITION
- TABLE 159 DISPOSABLE ENDOSCOPES MARKET: REGION FOOTPRINT
- TABLE 160 DISPOSABLE ENDOSCOPES MARKET: TYPE FOOTPRINT
- TABLE 161 DISPOSABLE ENDOSCOPES MARKET: APPLICATION FOOTPRINT
- TABLE 162 DISPOSABLE ENDOSCOPES MARKET: END-USER FOOTPRINT
- TABLE 163 DISPOSABLE ENDOSCOPES MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 164 DISPOSABLE ENDOSCOPES MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
- TABLE 165 DISPOSABLE ENDOSCOPES MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-OCTOBER 2025
- TABLE 166 DISPOSABLE ENDOSCOPES MARKET: DEALS, JANUARY 2022-OCTOBER 2025
- TABLE 167 DISPOSABLE ENDOSCOPES MARKET: EXPANSIONS, JANUARY 2022-OCTOBER 2025
- TABLE 168 AMBU A/S: COMPANY OVERVIEW
- TABLE 169 AMBU A/S: PRODUCTS OFFERED
- TABLE 170 AMBU A/S: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 171 AMBU A/S: EXPANSIONS, JANUARY 2022-JULY 2025
- TABLE 172 BOSTON SCIENTIFIC CORPORATION: COMPANY OVERVIEW
- TABLE 173 BOSTON SCIENTIFIC CORPORATION: PRODUCTS OFFERED
- TABLE 174 BOSTON SCIENTIFIC CORPORATION: PRODUCT APPROVALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 175 BOSTON SCIENTIFIC CORPORATION: DEALS, JANUARY 2022-JULY 2025
- TABLE 176 OLYMPUS CORPORATION: COMPANY OVERVIEW
- TABLE 177 OLYMPUS CORPORATION: PRODUCTS OFFERED
- TABLE 178 OLYMPUS CORPORATION: PRODUCT APPROVALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 179 OLYMPUS CORPORATION: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 180 HOYA CORPORATION: COMPANY OVERVIEW
- TABLE 181 HOYA CORPORATION: PRODUCTS OFFERED
- TABLE 182 HOYA CORPORATION: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 183 VERATHON INC.: COMPANY OVERVIEW
- TABLE 184 VERATHON INC.: PRODUCTS OFFERED
- TABLE 185 VERATHON INC.: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 186 KARL STORZ SE & CO. KG: COMPANY OVERVIEW
- TABLE 187 KARL STORZ SE & CO. KG: PRODUCTS OFFERED
- TABLE 188 KARL STORZ SE & CO. KG: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 189 KARL STORZ SE & CO. KG: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 190 HUNAN VATHIN MEDICAL INSTRUMENT CO., LTD.: COMPANY OVERVIEW
- TABLE 191 HUNAN VATHIN MEDICAL INSTRUMENT CO., LTD.: PRODUCTS OFFERED
- TABLE 192 HUNAN VATHIN MEDICAL INSTRUMENT CO., LTD.: DEALS, JANUARY 2022- SEPTEMBER 2025
- TABLE 193 INNOVEX MEDICAL CO., LTD.: COMPANY OVERVIEW
- TABLE 194 INNOVEX MEDICAL CO., LTD.: PRODUCTS OFFERED
- TABLE 195 SCIVITA MEDICAL: COMPANY OVERVIEW
- TABLE 196 SCIVITA MEDICAL: PRODUCTS OFFERED
- TABLE 197 GUANGZHOU RED PINE MEDICAL INSTRUMENT CO., LTD.: COMPANY OVERVIEW
- TABLE 198 GUANGZHOU RED PINE MEDICAL INSTRUMENT CO., LTD.: PRODUCTS OFFERED
- TABLE 199 THE COOPER COMPANIES, INC.: COMPANY OVERVIEW
- TABLE 200 ZHUHAI PUSEN MEDICAL TECHNOLOGY CO., LTD.: COMPANY OVERVIEW
- TABLE 201 UROVIU CORPORATION: COMPANY OVERVIEW
- TABLE 202 OTU MEDICAL: COMPANY OVERVIEW
- TABLE 203 GI VIEW LTD.: COMPANY OVERVIEW
- TABLE 204 TSC LIFE: COMPANY OVERVIEW
- TABLE 205 RICHARD WOLF GMBH: COMPANY OVERVIEW
- TABLE 206 DAICHUAN MEDICA: COMPANY OVERVIEW
- TABLE 207 NEOSCOPE INC.: COMPANY OVERVIEW
- TABLE 208 INTEGRATED ENDOSCOPY: COMPANY OVERVIEW
- TABLE 209 HUGER MEDICAL INSTRUMENT CO., LTD.: COMPANY OVERVIEW
- TABLE 210 XENOCOR: COMPANY OVERVIEW
- TABLE 211 MACROLUX MEDICAL TECHNOLOGY CO., LTD.: COMPANY OVERVIEW
- TABLE 212 SHENZHEN HUGEMED MEDICAL TECHNICAL DEVELOPMENT CO., LTD.: COMPANY OVERVIEW
- TABLE 213 ZHEJIANG GEYI MEDICAL DEVICES CO., LTD.: COMPANY OVERVIEW
- TABLE 214 DISPOSABLE ENDOSCOPES MARKET: STUDY ASSUMPTIONS
- TABLE 215 DISPOSABLE ENDOSCOPES MARKET: RISK ASSESSMENT ANALYSIS
List of Figures
- FIGURE 1 DISPOSABLE ENDOSCOPES MARKET SEGMENTATION & REGIONAL SCOPE
- FIGURE 2 KEY INSIGHTS & MARKET HIGHLIGHTS
- FIGURE 3 DISPOSABLE ENDOSCOPES MARKET, BY TYPE, 2025-2030 (USD MILLION)
- FIGURE 4 MAJOR STRATEGIES ADOPTED BY KEY PLAYERS IN DISPOSABLE ENDOSCOPES MARKET
- FIGURE 5 DISRUPTIVE TRENDS IMPACTING GROWTH OF DISPOSABLE ENDOSCOPES MARKET
- FIGURE 6 HIGH-GROWTH SEGMENTS & EMERGING FRONTIERS IN DISPOSABLE ENDOSCOPES MARKET, 2024
- FIGURE 7 NORTH AMERICA TO REGISTER HIGHEST GROWTH DURING FORECAST PERIOD
- FIGURE 8 RISING REQUIREMENT FOR ENDOSCOPY TO DIAGNOSE AND TREAT TARGET DISEASES TO DRIVE MARKET GROWTH
- FIGURE 9 HOSPITALS SEGMENT ACCOUNTED FOR LARGEST SHARE OF NORTH AMERICAN DISPOSABLE ENDOSCOPES MARKET IN 2024
- FIGURE 10 INDIA TO REGISTER HIGHEST GROWTH RATE DURING FORECAST PERIOD
- FIGURE 11 NORTH AMERICA TO GROW AT HIGHEST CAGR DURING FORECAST PERIOD
- FIGURE 12 DEVELOPING MARKETS TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
- FIGURE 13 DISPOSABLE ENDOSCOPES MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 14 DISPOSABLE ENDOSCOPES MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 15 DISPOSABLE ENDOSCOPES MARKET: SUPPLY CHAIN ANALYSIS
- FIGURE 16 DISPOSABLE ENDOSCOPES MARKET: VALUE CHAIN ANALYSIS
- FIGURE 17 DISPOSABLE ENDOSCOPES MARKET: ECOSYSTEM ANALYSIS
- FIGURE 18 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 19 DISPOSABLE ENDOSCOPES MARKET: INVESTMENT & FUNDING SCENARIO
- FIGURE 20 NUMBER OF PATENTS PUBLISHED (JANUARY 2015-OCTOBER 2025)
- FIGURE 21 TOP APPLICANT COUNTRIES/REGIONS FOR DISPOSABLE ENDOSCOPES (JANUARY 2015-OCTOBER 2025)
- FIGURE 22 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR DISPOSABLE ENDOSCOPES
- FIGURE 23 KEY BUYING CRITERIA FOR TOP THREE DISPOSABLE ENDOSCOPES
- FIGURE 24 DISPOSABLE ENDOSCOPES MARKET: GEOGRAPHIC SNAPSHOT
- FIGURE 25 NORTH AMERICA: DISPOSABLE ENDOSCOPES MARKET SNAPSHOT
- FIGURE 26 EUROPE: DISPOSABLE ENDOSCOPES MARKET SNAPSHOT
- FIGURE 27 REVENUE ANALYSIS OF KEY PLAYERS IN DISPOSABLE ENDOSCOPES MARKET (2020-2024)
- FIGURE 28 MARKET SHARE ANALYSIS OF KEY PLAYERS IN DISPOSABLE ENDOSCOPES MARKET (2024)
- FIGURE 29 DISPOSABLE ENDOSCOPES MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 30 DISPOSABLE ENDOSCOPES MARKET: COMPANY FOOTPRINT
- FIGURE 31 DISPOSABLE ENDOSCOPES MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 32 EV/EBITDA OF KEY VENDORS
- FIGURE 33 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 34 DISPOSABLE ENDOSCOPES MARKET: BRAND/PRODUCT COMPARATIVE ANALYSIS
- FIGURE 35 AMBU A/S: COMPANY SNAPSHOT (2024)
- FIGURE 36 BOSTON SCIENTIFIC CORPORATION: COMPANY SNAPSHOT (2024)
- FIGURE 37 OLYMPUS CORPORATION: COMPANY SNAPSHOT (2024)
- FIGURE 38 HOYA CORPORATION: COMPANY SNAPSHOT (2024)
- FIGURE 39 RESEARCH DESIGN
- FIGURE 40 PRIMARY SOURCES
- FIGURE 41 PRIMARY BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
- FIGURE 42 MARKET SIZE APPROACH: REVENUE SHARE ANALYSIS
- FIGURE 43 TOP-DOWN APPROACH
- FIGURE 44 BOTTOM-UP APPROACH
- FIGURE 45 IMPACT ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES (2025-2030)
- FIGURE 46 DISPOSABLE ENDOSCOPES MARKET: CAGR PROJECTIONS
- FIGURE 47 DATA TRIANGULATION METHODOLOGY











