 |
市場調查報告書
商品編碼
1880371
全球分子細胞遺傳學市場按產品、技術和應用分類-預測至2030年Molecular Cytogenetics Market by Product (Kits, Reagents, Probes, Instrument, Software, Services), Technique (FISH, CISH, Comparative Genomic Hybridization [Array-based, Standard]), Application (Cancer, Personalized Medicine) - Global Forecast to 2030 |
||||||
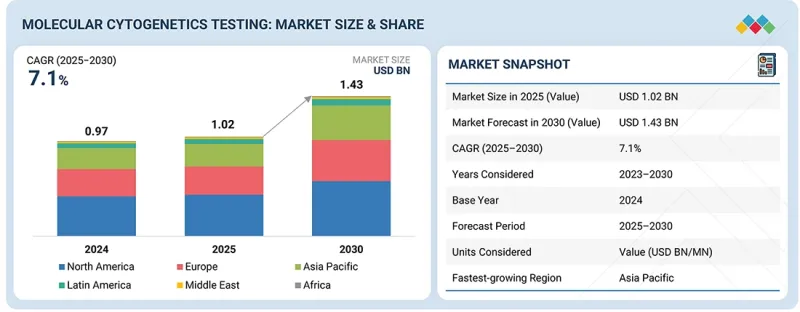
全球分子細胞遺傳學市場預計將從 2025 年的 10.2 億美元成長到 2030 年的 14.3 億美元,預測期內複合年成長率為 7.1%。
該市場的成長是由癌症和遺傳疾病(主要是老年人群)的日益普遍所驅動的,而分子細胞遺傳學檢測在準確診斷和疾病管理方面的日益普及也發揮了關鍵作用。
| 調查範圍 | |
|---|---|
| 調查期 | 2024-2030 |
| 基準年 | 2024 |
| 預測期 | 2025-2030 |
| 單元 | 10億美元 |
| 部分 | 產品/服務、技術、應用、最終用戶 |
| 目標區域 | 北美、歐洲、亞太地區、拉丁美洲、中東和非洲 |
此外,標靶治療的出現及其與個人化醫療的融合進一步推動了市場成長。然而,先進分子細胞遺傳學技術的高昂成本以及對專業技術人員的需求仍然是市場擴張的主要障礙。
“預計在預測期內,檢測套組細分市場將以最高的複合年成長率成長。”
在分子細胞遺傳學市場中,檢測套組細分市場憑藉其易用性、可重複性和快速準確檢測結果的能力,正以最高的複合年成長率成長。這些試劑盒,包括CGH晶片和FISH探針組,能夠幫助臨床和研究檢查室對染色體異常和基因突變進行標準化檢測。利用檢驗的檢測方法和整合的分析工具,檢測套組檢測套組可靠的高通量解決方案,協助腫瘤學、產前檢測和遺傳疾病診斷。其便利性、準確性和擴充性使其成為高效細胞遺傳學檢測的必備工具,從而推動了其在市場上的廣泛應用。
“腫瘤應用領域佔據了最大的市場佔有率。”
腫瘤應用領域佔據分子細胞遺傳學市場最大佔有率。這一成長主要歸因於全球癌症發生率的不斷上升,尤其是在老年人中,他們更容易患有惡性腫瘤。基因改變和染色體異常是腫瘤發生的主要原因,推動了腫瘤學領域對分子細胞遺傳學檢測的需求。螢光原位雜合反應(FISH)和比較基因組雜合反應(CGH)等技術能夠準確檢測這些異常,有助於準確診斷、腫瘤類型分類和疾病進展監測。此外,癌症研究投入的增加、醫療基礎設施的改善以及人們對早期檢測和基因分析益處的認知不斷提高,都促進了該領域在全球臨床和研究領域的強勁成長。
“預計美國在預測期內將以最高的複合年成長率成長。”
由於多種因素,美國預計將成為分子細胞遺傳學市場成長最快的國家。美國擁有許多大型細胞遺傳學公司、先進的研究基礎設施和大規模的患者群體,這些都是推動美國市場成長的主要因素。此外,分子細胞遺傳學檢測在腫瘤學、產前篩檢和遺傳疾病診斷領域的應用日益廣泛,也推動了市場需求。支持性的醫療保健法規、不斷增加的基因組研究經費以及對精準醫療日益成長的重視,也促進了美國分子細胞遺傳學市場的擴張。
此外,諸如FISH和CGH等分子細胞遺傳學技術的廣泛應用,能夠精準檢測染色體異常,正推動著分子細胞遺傳學的發展。政府和私人機構對生物醫學研究和腫瘤學的大量投入,促進了創新,並推動了尖端檢測平台的部署。該地區嚴格的法規結構確保了產品的品質和可靠性,進一步增強了人們對分子細胞遺傳學解決方案的信心,並鞏固了北美在全球的領先地位。
本報告分析了全球分子細胞遺傳學市場,提供了關鍵促進因素和限制因素、競爭格局和未來趨勢的資訊。
目錄
第1章 引言
第2章調查方法
第3章執行摘要
第4章 主要發現
- 北美分子細胞遺傳學市場按應用和國家分類
- 分子細胞遺傳學市場佔有率(按最終用戶分類)(2024 年)
- 分子細胞遺傳學市場:地域成長機遇
- 相互關聯的市場與跨產業機遇
- 一級/二級/三級公司的策略性舉措
- 創投/私募股權投資趨勢
第5章 市場概覽
- 介紹
- 市場動態
- 促進要素
- 抑制因素
- 機會
- 任務
- 未滿足的需求和閒置頻段
第6章 產業趨勢
- 影響客戶業務的趨勢/顛覆性因素
- 案例研究分析
- 技術分析
- 主要技術
- 互補技術
- 定價分析
- 主要企業分子和細胞遺傳學產品平均售價趨勢(2022-2024)
- 2022-2024年各地區家電平均售價趨勢
- 專利分析
- 調查方法
- 按文件類型分類的專利申請編號
- 主要專利列表
- 貿易分析
- HS編碼3822.00的進口資料(2020-2024年)
- HS編碼3822.00的出口資料(2020-2024年)
- 價值鏈分析
- 生態系分析
- 在生態系中的作用
- 新的經營模式和生態系統變化
- 波特五力分析
- 主要相關利益者和採購標準
- 關稅和監管分析
- 海關資料(HS編碼9027.50.80/3822.00)
- 監管環境
- 監管機構、政府機構和其他組織
- 永續性影響和監管政策舉措
- 重大會議和活動(2025-2026)
- 投資和資金籌措方案
- 人工智慧/生成式人工智慧對分子細胞遺傳學市場的影響
- 2025年美國關稅對分子細胞遺傳學市場的影響
- 介紹
- 主要關稅稅率
- 價格影響分析
- 對各國/地區的主要影響
- 對終端用戶產業的影響
7. 分子細胞遺傳學市場(依產品和服務分類)
- 介紹
- 試劑盒和試劑
- 檢測套組
- 探測
- 螢光親和性和試劑
- 其他試劑盒和試劑
- 消耗品
- 裝置
- 軟體和服務
第8章 依技術分類的分子細胞遺傳學市場
- 介紹
- 螢光原位雜合反應(FISH)
- 基於晶片的比較基因組雜合反應(ACGH)
- 顯色原位雜合反應(CISH)
- 其他技術
第9章 按應用分類的分子細胞遺傳學市場
- 介紹
- 遺傳性疾病
- 癌症
- 個人化醫療
- 其他用途
第10章 依最終用戶分類的分子細胞遺傳學市場
- 介紹
- 臨床和診斷檢查室
- 學術研究機構
- 製藥和生物技術公司
- 其他最終用戶
第11章 各地區分子細胞遺傳學市場
- 介紹
- 北美洲
- 北美宏觀經濟展望
- 美國
- 加拿大
- 歐洲
- 歐洲宏觀經濟展望
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 其他歐洲
- 亞太地區
- 亞太宏觀經濟展望
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 亞太其他地區
- 拉丁美洲
- 拉丁美洲宏觀經濟展望
- 巴西
- 墨西哥
- 其他拉丁美洲
- 中東
- 中東宏觀經濟展望
- 海灣合作理事會國家
- 其他中東地區
- 非洲
第12章 競爭格局
- 介紹
- 主要企業採取的策略概述
- 收入分析(2020-2024)
- 市佔率分析(2024 年)
- 公司估值和財務指標
- 品牌/產品對比
- F. HOFFMANN-LA ROCHE LTD.
- DANAHER CORPORATION
- AGILENT TECHNOLOGIES, INC.
- ABBOTT
- THERMO FISHER SCIENTIFIC INC.
- 企業評估矩陣:主要企業(2024)
- 公司評估矩陣:Start-Ups/中小企業(2024 年)
- 競爭場景
第13章:公司簡介
- 主要企業
- F. HOFFMANN-LA ROCHE LTD.
- DANAHER CORPORATION
- AGILENT TECHNOLOGIES, INC.
- ABBOTT
- THERMO FISHER SCIENTIFIC INC.
- ILLUMINA, INC.
- REVVITY
- PACBIO
- BIO-RAD LABORATORIES, INC.
- BIO-TECHNE
- GENE DX, LLC
- INSIGHT MOLECULAR DIAGNOSTICS INC.
- BIOVIEW
- 其他公司
- OXFORD GENE TECHNOLOGY IP LIMITED
- APPLIED SPECTRAL IMAGING
- CYTOTEST INC.
- KROMATID
- GENIAL GENETIC SOLUTIONS LTD.
- CYTOGNOMIX INC.
- METASYSTEMS
- SCIGENE CORPORATION
- BIOMODAL
- BIOCARE MEDICAL, LLC
- BIODOT
- ONCODNA
第14章附錄
The molecular cytogenetics market is expected to reach USD 1.43 billion in 2030 from USD 1.02 billion in 2025, at a CAGR of 7.1% during the forecast period. The market is driven by a rising prevalence of cancer and genetic disorders, mainly among older adults. The growing application of molecular cytogenetic tests for accurate diagnosis and disease management also plays a crucial role.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Product & Service, Technique, Applications, End User |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa |
Furthermore, the emergence of targeted therapies and their integration into personalized medicine are further driving market growth. Nonetheless, the high expenses associated with advanced molecular cytogenetic techniques and the requirement for skilled professionals continue to be major barriers to market expansion.

"Testing kits segment is expected to grow at the highest CAGR during the forecast period"
The testing kits segment is growing at the highest CAGR in the molecular cytogenetics market due to its ease of use, reproducibility, and ability to deliver rapid, accurate results. These kits, including CGH arrays and FISH probe panels, enable standardized detection of chromosomal abnormalities and genetic variations across clinical and research laboratories. By leveraging pre-validated assays and integrated analysis tools, testing kits offer reliable, high-throughput solutions that support oncology, prenatal testing, and genetic disorder diagnostics. Their convenience, accuracy, and scalability make them essential for efficient cytogenetic testing, driving their strong adoption in the market.
"Cancer application segment holds the largest share of the market"
The cancer applications segment accounts for the largest share of the molecular cytogenetics market. This growth is primarily driven by the rising incidence of cancer worldwide, particularly among the aging population, which is more susceptible to malignancies. Genetic changes and chromosomal abnormalities are major contributors to tumor development, increasing the demand for molecular cytogenetic testing in oncology. Techniques such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH) enable the precise detection of these abnormalities, facilitating accurate diagnosis, classification of tumor types, and monitoring of disease progression. Furthermore, increasing investments in cancer research, improvements in healthcare infrastructure, and growing awareness of the benefits of early detection and genetic profiling are contributing to the robust growth of this segment across clinical and research settings globally.
"US is expected to grow at the highest CAGR during the forecast period"
The US is experiencing the highest growth rate in the molecular cytogenetics market due to several factors. The presence of leading cytogenetics companies, advanced research infrastructure, and a large patient base are major drivers of market growth in the country. Additionally, rising adoption of molecular cytogenetic testing in oncology, prenatal screening, and genetic disorder diagnostics is fueling demand. Supportive healthcare regulations, increased funding for genomic research, and the growing focus on precision medicine further contribute to the expansion of the molecular cytogenetics market in the US.
Additionally, the widespread adoption of molecular cytogenetic techniques, such as FISH and CGH, for the accurate detection of chromosomal abnormalities has further supported the growth of molecular cytogenetics. Extensive government and private funding for biomedical research and oncology accelerates innovation and the deployment of cutting-edge testing platforms. The region's stringent regulatory framework ensures product quality and reliability, further strengthening trust in molecular cytogenetic solutions and solidifying North America's dominant position globally.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Tier 1 - 25%, Tier 2 - 35%, and Tier 3 - 40%
- By Designation: Managers - 45%, CXOs and Directors - 30%, and Executives - 25%
- By Region: North America - 35%, Europe - 25%, Asia Pacific - 15%, Latin America - 10%, the Middle East - 10%, and Africa - 5%
F. Hoffmann-La Roche Ltd. (Switzerland), Danaher (US), Agilent Technologies, Inc. (US), Abbott (US), Thermo Fisher Scientific Inc. (US), Illumina, Inc. (US), Revvity (US), PacBio (US), Bio-Rad Laboratories, Inc. (US), Bio-Techne (US), GeneDx, LLC (US), Insight Molecular Diagnostics Inc. (US), BioView (Israel), Oxford Gene Technology IP Limited (UK), Applied Spectral Imaging, Inc. (US), CytoTest Inc. (US), KROMATID (US), Genial Genetic Solutions Ltd. (UK), Cytognomix Inc. (Canada), MetaSystems (Germany), SciGene Corporation (US), Biomodal (UK), Biocare Medical, LLC (US), BioDot (US), and OncoDNA (Belgium) are some of the key companies offering molecular cytogenetics products.
Research Coverage
This research report categorizes the molecular cytogenetics market by product & service (kits & reagents [testing kits, probes, fluorescent affinity reagents, other kits & reagents], instruments, consumables, software & services), technique (comparative genomic hybridization [array-based comparative genomic hybridization, standard comparative genomic hybridization], fluorescence in-situ hybridization, chromogenic in-situ hybridization, other techniques), application (cancer, genetic disorders, personalized medicine and other applications), end user (clinical & research laboratories, academic & research institutes, pharmaceutical & biotechnology companies, other end users), and region (North America, Europe, Asia Pacific, Latin America, Middle East, And Africa).
The report's scope encompasses detailed information about the primary factors, including drivers, restraints, challenges, and opportunities, that influence the growth of the molecular cytogenetics market. A comprehensive analysis of key industry players has been performed to provide insights into their business overview, product portfolio, key strategies, new product launches, acquisitions, and recent developments related to the molecular cytogenetics market. This report also includes a competitive analysis of emerging startups in the molecular cytogenetics industry ecosystem.
Key Benefits of Buying the Report
The report will assist market leaders and new entrants by providing revenue estimates for the overall market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to position their businesses effectively and develop suitable go-to-market strategies. This report will enable stakeholders to grasp the market's pulse and offer information on key market drivers, restraints, opportunities, and challenges.
The report provides insights into the following pointers:
- Analysis of key drivers (increasing incidence of cancer and genetic disorders, growing focus on targeted cancer treatment, rapid growth in aging population and subsequent increase in prevalence of chronic diseases, and increasing penetration of molecular cytogenetics in clinical pathological testing), restraints (high cost of advanced instruments and unfavourable reimbursement scenario), opportunities (untapped emerging markets), and challenges (transition from FISH to array-based techniques) influencing the market growth
- Product Development/Innovation: Detailed insights into newly launched products and technological assessment of the molecular cytogenetics market
- Market Development: Comprehensive information about lucrative markets and analysis of the market across varied regions
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the molecular cytogenetics market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players, including F. Hoffman-La Roche Ltd (Switzerland), Danaher (US), Agilent Technologies, Inc. (US), Abbott (US), Thermo Fisher Scientific Inc. (US), Illumina, Inc. (US), among others offering products and services for molecular cytogenetics market. Other companies include Applied Spectral Imaging Inc. (US), Biomodal (UK), Biocare Medical, LLC (US), OncoDNA (Belgium), among others, for the molecular cytogenetics market
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
- 1.5 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key sources of secondary data
- 2.1.1.2 Key objectives of secondary research
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Breakdown of primaries
- 2.1.2.2 Key objectives of primary research
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 GLOBAL MOLECULAR CYTOGENETICS MARKET SIZE ESTIMATION, 2024
- 2.2.1.1 Revenue share analysis (bottom-up approach)
- 2.2.1.2 Secondary data & primary interviews
- 2.2.1.2.1 Insights of primary experts
- 2.2.1.3 MnM repository analysis
- 2.2.2 SEGMENTAL MARKET ASSESSMENT: TOP-DOWN APPROACH
- 2.2.1 GLOBAL MOLECULAR CYTOGENETICS MARKET SIZE ESTIMATION, 2024
- 2.3 MARKET GROWTH RATE PROJECTION
- 2.4 DATA TRIANGULATION
- 2.5 STUDY ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
- 3.1 KEY INSIGHTS & MARKET HIGHLIGHTS
- 3.2 STRATEGIC IMPERATIVES FOR STAKEHOLDERS
- 3.3 DISRUPTIVE TRENDS SHAPING MOLECULAR CYTOGENETICS MARKET
- 3.4 HIGH-GROWTH SEGMENTS & EMERGING FRONTIERS
- 3.5 GLOBAL MARKET SIZE, GROWTH RATE, AND FORECAST
4 PREMIUM INSIGHTS
- 4.1 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION AND COUNTRY
- 4.2 MOLECULAR CYTOGENETICS MARKET SHARE, BY END USER, 2024
- 4.3 MOLECULAR CYTOGENETICS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.4 INTERCONNECTED MARKETS & CROSS-SECTOR OPPORTUNITIES
- 4.5 STRATEGIC MOVIES BY TIER-1/2/3 PLAYERS
- 4.6 VC/PRIVATE EQUITY INVESTMENT TRENDS
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Advancement and adoption of high-resolution techniques
- 5.2.1.2 Growing focus on targeted cancer treatment
- 5.2.1.3 Increasing penetration of molecular cytogenetics in clinical pathological testing
- 5.2.2 RESTRAINTS
- 5.2.2.1 High cost of advanced instruments
- 5.2.2.2 Unfavorable reimbursement scenario for advanced diagnostic tests
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Untapped emerging markets
- 5.2.4 CHALLENGES
- 5.2.4.1 Transition from FISH to array-based molecular cytogenetic techniques in diagnostic and research communities
- 5.2.1 DRIVERS
- 5.3 UNMET NEEDS & WHITE SPACES
6 INDUSTRY TRENDS
- 6.1 TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- 6.2 CASE STUDY ANALYSIS
- 6.2.1 MOSAIC TETRASOMY 9P TO BE DETECTED BY CNV-SEQ IN PRENATAL DIAGNOSIS
- 6.2.2 PRENATAL DETECTION OF 15Q21.3 AND 16P11.2 MICRODUPLICATION SYNDROMES USING CNV-SEQ AND WES
- 6.2.3 INTEGRATED CNV-SEQ AND KARYOTYPING IN LARGE PRENATAL COHORT FOR CHROMOSOMAL ABNORMALITY DETECTION
- 6.3 TECHNOLOGY ANALYSIS
- 6.3.1 KEY TECHNOLOGIES
- 6.3.1.1 Optical genome mapping
- 6.3.1.2 Next-generation sequencing
- 6.3.2 COMPLEMENTARY TECHNOLOGIES
- 6.3.2.1 CRISPR-based editing
- 6.3.1 KEY TECHNOLOGIES
- 6.4 PRICING ANALYSIS
- 6.4.1 AVERAGE SELLING PRICE TREND OF MOLECULAR CYTOGENETIC PRODUCTS, BY KEY PLAYER, 2022-2024
- 6.4.2 AVERAGE SELLING PRICE TREND OF INSTRUMENTS, BY REGION, 2022-2024
- 6.5 PATENT ANALYSIS
- 6.5.1 METHODOLOGY
- 6.5.2 NUMBER OF PATENTS FILED, BY DOCUMENT TYPE
- 6.5.3 LIST OF KEY PATENTS
- 6.6 TRADE ANALYSIS
- 6.6.1 IMPORT DATA FOR HS CODE 3822.00, 2020-2024
- 6.6.2 EXPORT DATA FOR HS CODE 3822.00, 2020-2024
- 6.7 VALUE CHAIN ANALYSIS
- 6.8 ECOSYSTEM ANALYSIS
- 6.8.1 ROLE IN ECOSYSTEM
- 6.8.2 EMERGING BUSINESS MODELS & ECOSYSTEM SHIFTS
- 6.9 PORTER'S FIVE FORCES ANALYSIS
- 6.9.1 INTENSITY OF COMPETITIVE RIVALRY
- 6.9.2 BARGAINING POWER OF SUPPLIERS
- 6.9.3 BARGAINING POWER OF BUYERS
- 6.9.4 THREAT OF SUBSTITUTES
- 6.9.5 THREAT OF NEW ENTRANTS
- 6.10 KEY STAKEHOLDERS & BUYING CRITERIA
- 6.10.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 6.10.2 KEY BUYING CRITERIA
- 6.11 TARIFF & REGULATORY ANALYSIS
- 6.11.1 TARIFF DATA FOR HS CODES 9027.50.80 AND 3822.00
- 6.11.1.1 US
- 6.11.1.2 European Union
- 6.11.1.3 Asia Pacific
- 6.11.2 REGULATORY LANDSCAPE
- 6.11.2.1 North America
- 6.11.2.1.1 US
- 6.11.2.1.2 Canada
- 6.11.2.2 Europe
- 6.11.2.3 Asia Pacific
- 6.11.2.3.1 Japan
- 6.11.2.3.2 China
- 6.11.2.3.3 India
- 6.11.2.4 Latin America
- 6.11.2.4.1 Brazil
- 6.11.2.1 North America
- 6.11.3 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 6.11.4 SUSTAINABILITY IMPACT & REGULATORY POLICY INITIATIVES
- 6.11.1 TARIFF DATA FOR HS CODES 9027.50.80 AND 3822.00
- 6.12 KEY CONFERENCES & EVENTS, 2025-2026
- 6.13 INVESTMENT & FUNDING SCENARIO
- 6.14 IMPACT OF AI/GEN AI ON MOLECULAR CYTOGENETICS MARKET
- 6.15 IMPACT OF 2025 US TARIFF ON MOLECULAR CYTOGENETICS MARKET
- 6.15.1 INTRODUCTION
- 6.15.2 KEY TARIFF RATES
- 6.15.3 PRICE IMPACT ANALYSIS
- 6.15.4 KEY IMPACT ON COUNTRIES/REGIONS
- 6.15.4.1 North America
- 6.15.4.1.1 US
- 6.15.4.2 Europe
- 6.15.4.3 Asia Pacific
- 6.15.4.1 North America
- 6.15.5 IMPACT OF END-USE INDUSTRIES
- 6.15.5.1 Clinical & diagnostic laboratories
- 6.15.5.2 Academic & research institutes
- 6.15.5.3 Pharmaceutical & biotechnology companies
7 MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE
- 7.1 INTRODUCTION
- 7.2 KITS & REAGENTS
- 7.2.1 TESTING KITS
- 7.2.1.1 Standardized and integrated kit workflows to propel market growth
- 7.2.2 PROBES
- 7.2.2.1 Advances in oligonucleotide probe chemistry, multiplexing, and regulatory-driven product modernization to boost market
- 7.2.3 FLUORESCENT AFFINITY REAGENTS
- 7.2.3.1 Improved fluorochrome chemistry and pre-validated conjugates paired with advanced spectral imaging to drive growth
- 7.2.4 OTHER KITS & REAGENTS
- 7.2.1 TESTING KITS
- 7.3 CONSUMABLES
- 7.3.1 STANDARDIZATION AND EXPANDING CLINICAL APPLICATIONS TO PROMOTE MARKET GROWTH
- 7.4 INSTRUMENTS
- 7.4.1 AUTOMATION, AI-ENABLED IMAGING, AND INTEGRATED PLATFORMS TO DRIVE MARKET
- 7.5 SOFTWARE & SERVICES
- 7.5.1 AI INTEGRATION, WORKFLOW STANDARDIZATION, AND CLOUD-ENABLED DATA MANAGEMENT TO DRIVE MARKET
8 MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE
- 8.1 INTRODUCTION
- 8.2 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
- 8.2.1 INTEGRATION OF FISH AND ADVANCED IMAGING TECHNOLOGIES TO DRIVE GROWTH
- 8.3 ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH)
- 8.3.1 BETTER CLINICAL ACCEPTANCE AND IMPROVED TECHNOLOGICAL INTEGRATION TO PROPEL MARKET GROWTH
- 8.4 CHROMOGENIC IN SITU HYBRIDIZATION (CISH)
- 8.4.1 ENHANCED PROBE TECHNOLOGY AND BETTER WORKFLOW AUTOMATION TO BOOST MARKET GROWTH
- 8.5 OTHER TECHNIQUES
9 MOLECULAR CYTOGENETICS MARKET, BY APPLICATION
- 9.1 INTRODUCTION
- 9.2 GENETIC DISORDERS
- 9.2.1 EXPANDED CLINICAL GUIDELINES AND IMPROVED CMA/FISH TECHNOLOGIES TO PROPEL MARKET GROWTH
- 9.3 CANCER
- 9.3.1 RISING CANCER RESEARCH AND INCREASING USE OF AUTOMATED CYTOGENETIC PLATFORMS TO DRIVE MARKET
- 9.4 PERSONALIZED MEDICINE
- 9.4.1 STRATEGIC COLLABORATIONS AND BIOMARKER-DRIVEN CYTOGENETIC TESTING TO ACCELERATE MARKET GROWTH
- 9.5 OTHER APPLICATIONS
10 MOLECULAR CYTOGENETICS MARKET, BY END USER
- 10.1 INTRODUCTION
- 10.2 CLINICAL & DIAGNOSTIC LABORATORIES
- 10.2.1 INVESTMENT IN AUTOMATION AND INDUSTRY PARTNERSHIPS TO DRIVE MARKET GROWTH
- 10.3 ACADEMIC & RESEARCH INSTITUTES
- 10.3.1 STRATEGIC ACADEMIA-INDUSTRY COLLABORATIONS TO AUGMENT MARKET GROWTH
- 10.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
- 10.4.1 STRATEGIC ACQUISITIONS AND PRECISION MEDICINE INTEGRATION TO FUEL MARKET GROWTH
- 10.5 OTHER END USERS
11 MOLECULAR CYTOGENETICS MARKET, BY REGION
- 11.1 INTRODUCTION
- 11.2 NORTH AMERICA
- 11.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 11.2.2 US
- 11.2.2.1 US to dominate North American molecular cytogenetics market during study period
- 11.2.3 CANADA
- 11.2.3.1 Increasing cancer burden and expanding application of cytogenetics technology in oncology to drive market
- 11.3 EUROPE
- 11.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 11.3.2 GERMANY
- 11.3.2.1 Dynamic convergence of research excellence, clinical adoption, and industry collaboration to augment market growth
- 11.3.3 UK
- 11.3.3.1 Strong governmental support for genomics research and advanced healthcare infrastructure to aid market growth
- 11.3.4 FRANCE
- 11.3.4.1 Increasing government investment in pharmaceutical industry to support market growth
- 11.3.5 ITALY
- 11.3.5.1 Rising research activities in pharma R&D to boost market growth
- 11.3.6 SPAIN
- 11.3.6.1 Well-established network of research centers and universities to boost clinical and diagnostic research
- 11.3.7 REST OF EUROPE
- 11.4 ASIA PACIFIC
- 11.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 11.4.2 CHINA
- 11.4.2.1 Development of advanced healthcare infrastructure and government support for genomics research to propel growth
- 11.4.3 JAPAN
- 11.4.3.1 Technological adoption and increased clinical programs to favor market growth
- 11.4.4 INDIA
- 11.4.4.1 Rising awareness of cancer and genetic disorders to support market growth
- 11.4.5 AUSTRALIA
- 11.4.5.1 Progressive translational research, advanced diagnostic integration, and cross-institutional collaborations to fuel growth
- 11.4.6 SOUTH KOREA
- 11.4.6.1 Technological innovation and high demand for precision medicines to aid market growth
- 11.4.7 REST OF ASIA PACIFIC
- 11.5 LATIN AMERICA
- 11.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 11.5.2 BRAZIL
- 11.5.2.1 Increased number of public-private collaborations to focus on strategic market expansions
- 11.5.3 MEXICO
- 11.5.3.1 Strengthening genomic infrastructure and technology integration to support market growth
- 11.5.4 REST OF LATIN AMERICA
- 11.6 MIDDLE EAST
- 11.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST
- 11.6.2 GCC COUNTRIES
- 11.6.2.1 Kingdom of Saudi Arabia
- 11.6.2.1.1 Growing healthcare expenditure to boost market growth
- 11.6.2.2 UAE
- 11.6.2.2.1 Rising government support and incorporation of ISH technologies by hospitals to aid market growth
- 11.6.2.3 Rest of GCC countries
- 11.6.2.1 Kingdom of Saudi Arabia
- 11.6.3 REST OF MIDDLE EAST
- 11.7 AFRICA
- 11.7.1 GROWING MARKET FOR PHARMACEUTICALS AND INCREASING DEMAND FOR GENETIC TESTING TO PROPEL GROWTH
- 11.7.2 MACROECONOMIC OUTLOOK FOR AFRICA
12 COMPETITIVE LANDSCAPE
- 12.1 INTRODUCTION
- 12.2 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS
- 12.3 REVENUE ANALYSIS, 2020-2024
- 12.4 MARKET SHARE ANALYSIS, 2024
- 12.5 COMPANY VALUATION & FINANCIAL METRICS
- 12.5.1 FINANCIAL METRICS
- 12.5.2 COMPANY VALUATION
- 12.6 BRAND/PRODUCT COMPARISON
- 12.6.1 F. HOFFMANN-LA ROCHE LTD.
- 12.6.2 DANAHER CORPORATION
- 12.6.3 AGILENT TECHNOLOGIES, INC.
- 12.6.4 ABBOTT
- 12.6.5 THERMO FISHER SCIENTIFIC INC.
- 12.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 12.7.1 STARS
- 12.7.2 EMERGING LEADERS
- 12.7.3 PERVASIVE PLAYERS
- 12.7.4 PARTICIPANTS
- 12.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 12.7.5.1 Company footprint
- 12.7.5.2 Region footprint
- 12.7.5.3 Product & service footprint
- 12.7.5.4 Technique footprint
- 12.7.5.5 Application footprint
- 12.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 12.8.1 PROGRESSIVE COMPANIES
- 12.8.2 RESPONSIVE COMPANIES
- 12.8.3 DYNAMIC COMPANIES
- 12.8.4 STARTING BLOCKS
- 12.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 12.8.5.1 Detailed list of key startups/SMEs
- 12.8.5.2 Competitive benchmarking of key startups/SMEs
- 12.9 COMPETITIVE SCENARIO
- 12.9.1 PRODUCT LAUNCHES & APPROVALS
- 12.9.2 DEALS
- 12.9.3 EXPANSIONS
- 12.9.4 OTHER DEVELOPMENTS
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- 13.1.1 F. HOFFMANN-LA ROCHE LTD.
- 13.1.1.1 Business overview
- 13.1.1.2 Products/Services offered
- 13.1.1.3 Recent developments
- 13.1.1.3.1 Product launches & approvals
- 13.1.1.4 MnM view
- 13.1.1.4.1 Key strengths
- 13.1.1.4.2 Strategic choices
- 13.1.1.4.3 Weaknesses & competitive threats
- 13.1.2 DANAHER CORPORATION
- 13.1.2.1 Business overview
- 13.1.2.2 Products/Services offered
- 13.1.2.3 Recent developments
- 13.1.2.3.1 Product launches
- 13.1.2.3.2 Deals
- 13.1.2.4 MnM view
- 13.1.2.4.1 Key strengths
- 13.1.2.4.2 Strategic choices
- 13.1.2.4.3 Weaknesses & competitive threats
- 13.1.3 AGILENT TECHNOLOGIES, INC.
- 13.1.3.1 Business overview
- 13.1.3.2 Products/Services offered
- 13.1.3.3 Recent developments
- 13.1.3.3.1 Product launches
- 13.1.3.3.2 Deals
- 13.1.3.3.3 Other developments
- 13.1.3.4 MnM view
- 13.1.3.4.1 Key strengths
- 13.1.3.4.2 Strategic choices
- 13.1.3.4.3 Weaknesses & competitive threats
- 13.1.4 ABBOTT
- 13.1.4.1 Business overview
- 13.1.4.2 Products/Services offered
- 13.1.4.3 MnM view
- 13.1.4.3.1 Key strengths
- 13.1.4.3.2 Strategic choices
- 13.1.4.3.3 Weaknesses & competitive threats
- 13.1.5 THERMO FISHER SCIENTIFIC INC.
- 13.1.5.1 Business overview
- 13.1.5.2 Products/Services offered
- 13.1.5.3 Recent developments
- 13.1.5.3.1 Product launches
- 13.1.5.3.2 Deals
- 13.1.5.4 MnM view
- 13.1.5.4.1 Key strengths
- 13.1.5.4.2 Strategic choices
- 13.1.5.4.3 Weaknesses & competitive threats
- 13.1.6 ILLUMINA, INC.
- 13.1.6.1 Business overview
- 13.1.6.2 Products/Services offered
- 13.1.6.3 Recent developments
- 13.1.6.3.1 Deals
- 13.1.6.3.2 Expansions
- 13.1.7 REVVITY
- 13.1.7.1 Business overview
- 13.1.7.2 Products/Services offered
- 13.1.7.3 Recent developments
- 13.1.7.3.1 Product launches
- 13.1.7.3.2 Deals
- 13.1.8 PACBIO
- 13.1.8.1 Business overview
- 13.1.8.2 Products/Services offered
- 13.1.8.3 Recent developments
- 13.1.8.3.1 Deals
- 13.1.8.3.2 Expansions
- 13.1.9 BIO-RAD LABORATORIES, INC.
- 13.1.9.1 Business overview
- 13.1.9.2 Products/Services offered
- 13.1.10 BIO-TECHNE
- 13.1.10.1 Business overview
- 13.1.10.2 Products/Services offered
- 13.1.10.3 Recent developments
- 13.1.10.3.1 Product launches
- 13.1.10.3.2 Deals
- 13.1.11 GENE DX, LLC
- 13.1.11.1 Business overview
- 13.1.11.2 Products/Services offered
- 13.1.11.3 Recent developments
- 13.1.11.3.1 Deals
- 13.1.12 INSIGHT MOLECULAR DIAGNOSTICS INC.
- 13.1.12.1 Business overview
- 13.1.12.2 Products/Services offered
- 13.1.13 BIOVIEW
- 13.1.13.1 Business overview
- 13.1.13.2 Products/Services offered
- 13.1.1 F. HOFFMANN-LA ROCHE LTD.
- 13.2 OTHER PLAYERS
- 13.2.1 OXFORD GENE TECHNOLOGY IP LIMITED
- 13.2.2 APPLIED SPECTRAL IMAGING
- 13.2.3 CYTOTEST INC.
- 13.2.4 KROMATID
- 13.2.5 GENIAL GENETIC SOLUTIONS LTD.
- 13.2.6 CYTOGNOMIX INC.
- 13.2.7 METASYSTEMS
- 13.2.8 SCIGENE CORPORATION
- 13.2.9 BIOMODAL
- 13.2.10 BIOCARE MEDICAL, LLC
- 13.2.11 BIODOT
- 13.2.12 ONCODNA
14 APPENDIX
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.3 CUSTOMIZATION OPTIONS
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS
List of Tables
- TABLE 1 MOLECULAR CYTOGENETICS MARKET: INCLUSIONS & EXCLUSIONS
- TABLE 2 IMPACT ANALYSIS OF SUPPLY- AND DEMAND-SIDE FACTORS
- TABLE 3 MOLECULAR CYTOGENETICS MARKET: RISK ANALYSIS
- TABLE 4 IMPACT ANALYSIS OF MOLECULAR CYTOGENETICS MARKET DYNAMICS
- TABLE 5 AVERAGE SELLING PRICE TREND OF MOLECULAR CYTOGENETIC PRODUCTS, BY KEY PLAYER, 2022-2024 (USD)
- TABLE 6 AVERAGE SELLING PRICE TREND OF INSTRUMENTS, BY REGION, 2022-2024 (USD)
- TABLE 7 AVERAGE SELLING PRICE TREND OF KITS, BY REGION, 2022-2024 (USD)
- TABLE 8 AVERAGE SELLING PRICE TREND OF REAGENTS, BY REGION, 2022-2024 (USD)
- TABLE 9 NUMBER OF PATENTS FILED IN MOLECULAR CYTOGENETICS MARKET, BY DOCUMENT TYPE, 2014-2024
- TABLE 10 LIST OF KEY PATENTS IN MOLECULAR CYTOGENETICS MARKET, 2023-2025
- TABLE 11 IMPORT DATA FOR HS CODE 3822.00, BY COUNTRY, 2020-2024 (USD THOUSAND)
- TABLE 12 EXPORT DATA FOR HS CODE 3822.00, BY COUNTRY, 2020-2024 (USD THOUSAND)
- TABLE 13 MOLECULAR CYTOGENETICS MARKET: ROLE IN ECOSYSTEM
- TABLE 14 MOLECULAR CYTOGENETICS MARKET: PORTER'S FIVE FORCES
- TABLE 15 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT & SERVICE
- TABLE 16 KEY BUYING CRITERIA, BY END USER
- TABLE 17 TARIFF DATA FOR HS CODES 9027.50.80 AND 3822.00
- TABLE 18 US: CLASSIFICATION OF IN VITRO DIAGNOSTIC DEVICES
- TABLE 19 EUROPE: CLASSIFICATION OF IN VITRO DIAGNOSTIC DEVICES
- TABLE 20 JAPAN: CLASSIFICATION OF IN VITRO DIAGNOSTIC REAGENTS
- TABLE 21 JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 22 CHINA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 23 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 24 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 25 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 26 REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 27 LIST OF KEY CONFERENCES & EVENTS IN MOLECULAR CYTOGENETICS MARKET, JANUARY 2025-DECEMBER 2026
- TABLE 28 KEY PLAYERS IMPLEMENTING AI/GEN AI IN MOLECULAR CYTOGENETICS MARKET
- TABLE 29 US ADJUSTED RECIPROCAL TARIFF RATES
- TABLE 30 EXPORTS AND IMPORTS, BY REGION
- TABLE 31 KEY PRODUCT-RELATED TARIFF: HS CODES FOR PRODUCTS RELEVANT TO MOLECULAR CYTOGENETICS
- TABLE 32 CRITICAL COMPONENTS LIKELY EXPOSED TO TARIFF CHANGES
- TABLE 33 MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 34 MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 35 MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 36 NORTH AMERICA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 37 EUROPE: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 38 ASIA PACIFIC: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 39 LATIN AMERICA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 40 MIDDLE EAST: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 41 GCC COUNTRIES: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 42 MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 43 NORTH AMERICA: MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 44 EUROPE: MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 45 ASIA PACIFIC: MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 46 LATIN AMERICA: MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 47 MIDDLE EAST: MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 48 GCC COUNTRIES: MOLECULAR CYTOGENETICS TESTING KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 49 MOLECULAR CYTOGENETICS PROBES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 50 NORTH AMERICA: MOLECULAR CYTOGENETICS PROBES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 51 EUROPE: MOLECULAR CYTOGENETICS PROBES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 52 ASIA PACIFIC: MOLECULAR CYTOGENETICS PROBES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 53 LATIN AMERICA: MOLECULAR CYTOGENETICS PROBES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 54 MIDDLE EAST: MOLECULAR CYTOGENETICS PROBES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 55 GCC COUNTRIES: MOLECULAR CYTOGENETICS PROBES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 56 MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 57 NORTH AMERICA: MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 58 EUROPE: MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 59 ASIA PACIFIC: MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 60 LATIN AMERICA: MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 61 MIDDLE EAST: MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 62 GCC COUNTRIES: MOLECULAR CYTOGENETICS FLUORESCENT AFFINITY REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 63 OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 64 NORTH AMERICA: OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 65 EUROPE: OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 66 ASIA PACIFIC: OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 67 LATIN AMERICA: OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 68 MIDDLE EAST: OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 69 GCC COUNTRIES: OTHER MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 70 MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 71 NORTH AMERICA: MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 72 EUROPE: MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 73 ASIA PACIFIC: MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 74 LATIN AMERICA: MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 75 MIDDLE EAST: MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 76 GCC COUNTRIES: MOLECULAR CYTOGENETICS CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 77 MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 78 NORTH AMERICA: MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 79 EUROPE: MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 80 ASIA PACIFIC: MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 81 LATIN AMERICA: MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 82 MIDDLE EAST: MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 83 GCC COUNTRIES: MOLECULAR CYTOGENETICS INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 84 MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 85 NORTH AMERICA: MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 86 EUROPE: MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 87 ASIA PACIFIC: MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 88 LATIN AMERICA: MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 89 MIDDLE EAST: MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 90 GCC COUNTRIES: MOLECULAR CYTOGENETICS SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 91 MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 92 MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY REGION, 2023-2030 (USD MILLION)
- TABLE 93 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 94 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 95 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 96 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 97 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY REGION, 2023-2030 (USD MILLION)
- TABLE 98 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION (FISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 99 MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY REGION, 2023-2030 (USD MILLION)
- TABLE 100 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 101 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 102 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 103 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 104 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY REGION, 2023-2030 (USD MILLION)
- TABLE 105 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR ARRAY-BASED COMPARATIVE GENOMIC HYBRIDIZATION (ACGH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 106 MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY REGION, 2023-2030 (USD MILLION)
- TABLE 107 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 108 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 109 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 110 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 111 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 112 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR CHROMOGENIC IN SITU HYBRIDIZATION (CISH), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 113 MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 114 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 115 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 116 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 117 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 118 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 119 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR OTHER TECHNIQUES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 120 MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 121 MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 122 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 123 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 124 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 125 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 126 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 127 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR GENETIC DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 128 MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY REGION, 2023-2030 (USD MILLION)
- TABLE 129 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 130 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 131 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 132 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 133 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY REGION, 2023-2030 (USD MILLION)
- TABLE 134 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR CANCER, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 135 MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 136 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 137 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 138 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 139 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 140 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 141 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR PERSONALIZED MEDICINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 142 MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 143 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 144 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 145 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 146 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 147 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 148 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 149 MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 150 MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 151 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 152 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 153 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 154 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 155 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 156 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR CLINICAL & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 157 MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 158 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 159 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 160 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 161 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 162 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 163 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 164 MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 165 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 166 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 167 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 168 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 169 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 170 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 171 MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 172 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 173 EUROPE: MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 174 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 175 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 176 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 177 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 178 MOLECULAR CYTOGENETICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 179 NORTH AMERICA: KEY MACROINDICATORS
- TABLE 180 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 181 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 182 NORTH AMERICA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 183 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 184 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 185 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 186 US: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 187 US: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 188 US: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 189 US: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 190 US: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 191 CANADA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 192 CANADA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 193 CANADA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 194 CANADA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 195 CANADA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 196 EUROPE: KEY MACROINDICATORS
- TABLE 197 EUROPE: MOLECULAR CYTOGENETICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 198 EUROPE: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 199 EUROPE: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 200 EUROPE: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 201 EUROPE: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 202 EUROPE: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 203 GERMANY: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 204 GERMANY: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 205 GERMANY: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 206 GERMANY: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 207 GERMANY: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 208 UK: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 209 UK: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 210 UK: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 211 UK: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 212 UK: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 213 FRANCE: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 214 FRANCE: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 215 FRANCE: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 216 FRANCE: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 217 FRANCE: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 218 ITALY: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 219 ITALY: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 220 ITALY: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 221 ITALY: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 222 ITALY: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 223 SPAIN: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 224 SPAIN: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 225 SPAIN: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 226 SPAIN: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 227 SPAIN: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 228 REST OF EUROPE: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 229 REST OF EUROPE: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 230 REST OF EUROPE: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 231 REST OF EUROPE: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 232 REST OF EUROPE: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 233 ASIA PACIFIC: KEY MACROINDICATORS
- TABLE 234 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 235 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 236 ASIA PACIFIC: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 237 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 238 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 239 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 240 CHINA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 241 CHINA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 242 CHINA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 243 CHINA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 244 CHINA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 245 JAPAN: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 246 JAPAN: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 247 JAPAN: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 248 JAPAN: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 249 JAPAN: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 250 INDIA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 251 INDIA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 252 INDIA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 253 INDIA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 254 INDIA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 255 AUSTRALIA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 256 AUSTRALIA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 257 AUSTRALIA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 258 AUSTRALIA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 259 AUSTRALIA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 260 SOUTH KOREA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 261 SOUTH KOREA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 262 SOUTH KOREA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 263 SOUTH KOREA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 264 SOUTH KOREA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 265 REST OF ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 266 REST OF ASIA PACIFIC: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 267 REST OF ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 268 REST OF ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 269 REST OF ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 270 LATIN AMERICA: KEY MACROINDICATORS
- TABLE 271 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 272 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 273 LATIN AMERICA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 274 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 275 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 276 LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 277 BRAZIL: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 278 BRAZIL: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 279 BRAZIL: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 280 BRAZIL: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 281 BRAZIL: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 282 MEXICO: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 283 MEXICO: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 284 MEXICO: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 285 MEXICO: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 286 MEXICO: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 287 REST OF LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 288 REST OF LATIN AMERICA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 289 REST OF LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 290 REST OF LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 291 REST OF LATIN AMERICA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 292 MIDDLE EAST: KEY MACROINDICATORS
- TABLE 293 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 294 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 295 MIDDLE EAST: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 296 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 297 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 298 MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 299 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 300 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 301 GCC COUNTRIES: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 302 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 303 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 304 GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 305 KINGDOM OF SAUDI ARABIA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 306 KINGDOM OF SAUDI ARABIA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 307 KINGDOM OF SAUDI ARABIA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 308 KINGDOM OF SAUDI ARABIA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 309 KINGDOM OF SAUDI ARABIA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 310 UAE: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 311 UAE: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 312 UAE: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 313 UAE: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 314 UAE: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 315 REST OF GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 316 REST OF GCC COUNTRIES: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 317 REST OF GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 318 REST OF GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 319 REST OF GCC COUNTRIES: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 320 REST OF MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 321 REST OF MIDDLE EAST: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 322 REST OF MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 323 REST OF MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 324 REST OF MIDDLE EAST: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 325 AFRICA: KEY MACROINDICATORS
- TABLE 326 AFRICA: MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 327 AFRICA: MOLECULAR CYTOGENETICS KITS & REAGENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 328 AFRICA: MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2023-2030 (USD MILLION)
- TABLE 329 AFRICA: MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 330 AFRICA: MOLECULAR CYTOGENETICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 331 OVERVIEW OF STRATEGIES DEPLOYED BY KEY PLAYERS IN MOLECULAR CYTOGENETICS MARKET, JANUARY 2022-SEPTEMBER 2025
- TABLE 332 MOLECULAR CYTOGENETICS MARKET: DEGREE OF COMPETITION
- TABLE 333 MOLECULAR CYTOGENETICS MARKET: REGION FOOTPRINT
- TABLE 334 MOLECULAR CYTOGENETICS MARKET: PRODUCT & SERVICE FOOTPRINT
- TABLE 335 MOLECULAR CYTOGENETICS MARKET: TECHNIQUE FOOTPRINT
- TABLE 336 MOLECULAR CYTOGENETICS MARKET: APPLICATION FOOTPRINT
- TABLE 337 MOLECULAR CYTOGENETICS MARKET: DETAILED LIST OF KEY STARTUPS/SME PLAYERS
- TABLE 338 MOLECULAR CYTOGENETICS MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SME PLAYERS, BY PRODUCT & SERVICE AND REGION
- TABLE 339 MOLECULAR CYTOGENETICS MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 340 MOLECULAR CYTOGENETICS MARKET: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 341 MOLECULAR CYTOGENETICS MARKET: EXPANSIONS, JANUARY 2022-SEPTEMBER 2025
- TABLE 342 MOLECULAR CYTOGENETICS MARKET: OTHER DEVELOPMENTS, JANUARY 2022-SEPTEMBER 2025
- TABLE 343 F. HOFFMANN-LA ROCHE LTD.: COMPANY OVERVIEW
- TABLE 344 F. HOFFMANN-LA ROCHE LTD.: PRODUCTS/SERVICES OFFERED
- TABLE 345 F. HOFFMANN-LA ROCHE LTD.: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 346 DANAHER CORPORATION: COMPANY OVERVIEW
- TABLE 347 DANAHER CORPORATION: PRODUCTS/SERVICES OFFERED
- TABLE 348 DANAHER CORPORATION: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 349 DANAHER CORPORATION: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 350 AGILENT TECHNOLOGIES, INC.: COMPANY OVERVIEW
- TABLE 351 AGILENT TECHNOLOGIES, INC.: PRODUCTS/SERVICES OFFERED
- TABLE 352 AGILENT TECHNOLOGIES, INC.: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 353 AGILENT TECHNOLOGIES, INC.: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 354 AGILENT TECHNOLOGIES, INC.: OTHER DEVELOPMENTS, JANUARY 2022-SEPTEMBER 2025
- TABLE 355 ABBOTT: COMPANY OVERVIEW
- TABLE 356 ABBOTT: PRODUCTS/SERVICES OFFERED
- TABLE 357 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
- TABLE 358 THERMO FISHER SCIENTIFIC INC.: PRODUCTS/SERVICES OFFERED
- TABLE 359 THERMO FISHER SCIENTIFIC INC.: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 360 THERMO FISHER SCIENTIFIC INC.: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 361 ILLUMINA, INC.: COMPANY OVERVIEW
- TABLE 362 ILLUMINA, INC.: PRODUCTS/SERVICES OFFERED
- TABLE 363 ILLUMINA, INC.: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 364 ILLUMINA, INC.: EXPANSIONS, JANUARY 2022-SEPTEMBER 2025
- TABLE 365 REVVITY: COMPANY OVERVIEW
- TABLE 366 REVVITY: PRODUCTS/SERVICES OFFERED
- TABLE 367 REVVITY: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 368 REVVITY: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 369 PACBIO: COMPANY OVERVIEW
- TABLE 370 PACBIO: PRODUCTS/SERVICES OFFERED
- TABLE 371 PACBIO: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 372 PACBIO: EXPANSIONS, JANUARY 2022-SEPTEMBER 2025
- TABLE 373 BIO-RAD LABORATORIES, INC.: COMPANY OVERVIEW
- TABLE 374 BIO-RAD LABORATORIES, INC.: PRODUCTS/SERVICES OFFERED
- TABLE 375 BIO-TECHNE: COMPANY OVERVIEW
- TABLE 376 BIO-TECHNE: PRODUCTS/SERVICES OFFERED
- TABLE 377 BIO-TECHNE: PRODUCT LAUNCHES, JANUARY 2022-SEPTEMBER 2025
- TABLE 378 BIO-TECHNE: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 379 GENE DX, LLC: COMPANY OVERVIEW
- TABLE 380 GENE DX, LLC: PRODUCTS/SERVICES OFFERED
- TABLE 381 GENE DX, LLC: DEALS, JANUARY 2022-SEPTEMBER 2025
- TABLE 382 INSIGHT MOLECULAR DIAGNOSTICS INC.: COMPANY OVERVIEW
- TABLE 383 INISIGHT MOLECULAR DIAGNOSTICS INC.: PRODUCTS/SERVICES OFFERED
- TABLE 384 BIOVIEW: COMPANY OVERVIEW
- TABLE 385 BIOVIEW: PRODUCTS/SERVICES OFFERED
- TABLE 386 OXFORD GENE TECHNOLOGY IP LIMITED: COMPANY OVERVIEW
- TABLE 387 APPLIED SPECTRAL IMAGING: COMPANY OVERVIEW
- TABLE 388 CYTOTEST INC.: COMPANY OVERVIEW
- TABLE 389 KROMATID: COMPANY OVERVIEW
- TABLE 390 GENIAL GENETIC SOLUTIONS LTD.: COMPANY OVERVIEW
- TABLE 391 CYTOGNOMIX INC.: COMPANY OVERVIEW
- TABLE 392 METASYSTEMS: COMPANY OVERVIEW
- TABLE 393 SCIGENE CORPORATION: COMPANY OVERVIEW
- TABLE 394 BIOMODAL: COMPANY OVERVIEW
- TABLE 395 BIOCARE MEDICAL, LLC: COMPANY OVERVIEW
- TABLE 396 BIODOT: COMPANY OVERVIEW
- TABLE 397 ONCODNA: COMPANY OVERVIEW
List of Figures
- FIGURE 1 MOLECULAR CYTOGENETICS MARKET SEGMENTATION & REGIONAL SCOPE
- FIGURE 2 MOLECULAR CYTOGENETICS MARKET: YEARS CONSIDERED
- FIGURE 3 MOLECULAR CYTOGENETICS MARKET: RESEARCH DESIGN
- FIGURE 4 MOLECULAR CYTOGENETICS MARKET: BREAKDOWN OF PRIMARIES (SUPPLY- AND DEMAND-SIDE PARTICIPANTS)
- FIGURE 5 MOLECULAR CYTOGENETICS MARKET SIZE ESTIMATION (SUPPLY-SIDE ANALYSIS), 2024
- FIGURE 6 MOLECULAR CYTOGENETICS MARKET: COMPANY REVENUE ANALYSIS-BASED ESTIMATION (BOTTOM-UP APPROACH), 2024
- FIGURE 7 REVENUE ANALYSIS: ILLUSTRATIVE EXAMPLE OF F. HOFFMANN-LA ROCHE LTD. (2024)
- FIGURE 8 MOLECULAR CYTOGENETICS MARKET: INSIGHTS FROM KEY PRIMARIES
- FIGURE 9 MOLECULAR CYTOGENETICS MARKET SIZE ESTIMATION: TOP-DOWN APPROACH
- FIGURE 10 MOLECULAR CYTOGENETICS MARKET: CAGR PROJECTIONS
- FIGURE 11 MOLECULAR CYTOGENETICS MARKET: DATA TRIANGULATION
- FIGURE 12 MOLECULAR CYTOGENETICS MARKET, BY PRODUCT & SERVICE, 2025 VS. 2030 (USD MILLION)
- FIGURE 13 MOLECULAR CYTOGENETICS MARKET, BY TECHNIQUE, 2025 VS. 2030 (USD MILLION)
- FIGURE 14 MOLECULAR CYTOGENETICS MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 15 MOLECULAR CYTOGENETICS MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- FIGURE 16 MOLECULAR CYTOGENETICS MARKET: REGIONAL ANALYSIS
- FIGURE 17 SNAPSHOT: GLOBAL MARKET SIZE, GROWTH RATE, AND FORECAST (USD MILLION)
- FIGURE 18 US AND GENETIC DISORDERS COMMANDED LARGEST NORTH AMERICAN MARKET SHARE IN 2024
- FIGURE 19 CLINICAL & DIAGNOSTIC LABORATORIES ACCOUNTED FOR LARGEST MARKET SHARE IN 2024
- FIGURE 20 ASIA PACIFIC TO BE FASTEST-GROWING REGION IN GLOBAL MOLECULAR CYTOGENETICS MARKET DURING STUDY PERIOD
- FIGURE 21 MOLECULAR CYTOGENETICS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 22 NUMBER OF CANCER CASES IN US, 2019-2024
- FIGURE 23 MOLECULAR CYTOGENETICS MARKET: TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- FIGURE 24 AVERAGE SELLING PRICE OF INSTRUMENTS, BY KEY PLAYER, 2024 (USD)
- FIGURE 25 AVERAGE SELLING PRICE OF KITS, BY KEY PLAYER, 2024 (USD)
- FIGURE 26 AVERAGE SELLING PRICE OF REAGENTS, BY KEY PLAYER, 2024 (USD)
- FIGURE 27 AVERAGE SELLING PRICE OF INSTRUMENTS, BY REGION, 2024 (USD)
- FIGURE 28 AVERAGE SELLING PRICE OF KITS, BY REGION, 2024 (USD)
- FIGURE 29 AVERAGE SELLING PRICE OF REAGENTS, BY REGION, 2024 (USD)
- FIGURE 30 PATENT APPLICATIONS IN MOLECULAR CYTOGENETICS MARKET, JANUARY 2014-DECEMBER 2024
- FIGURE 31 MOLECULAR CYTOGENETICS MARKET: VALUE CHAIN ANALYSIS
- FIGURE 32 MOLECULAR CYTOGENETICS MARKET: ECOSYSTEM ANALYSIS
- FIGURE 33 MOLECULAR CYTOGENETICS MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 34 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT & SERVICE
- FIGURE 35 KEY BUYING CRITERIA, BY END USER
- FIGURE 36 US: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 37 CANADA: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 38 EUROPE: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 39 JAPAN: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 40 INDIA: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 41 BRAZIL: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 42 FUNDING AND NUMBER OF DEALS IN MOLECULAR CYTOGENETICS MARKET, 2022-2025 (USD MILLION)
- FIGURE 43 IMPACT OF AI/GEN AI ON MOLECULAR CYTOGENETICS MARKET
- FIGURE 44 NORTH AMERICA: MOLECULAR CYTOGENETICS MARKET SNAPSHOT
- FIGURE 45 ASIA PACIFIC: MOLECULAR CYTOGENETICS MARKET SNAPSHOT
- FIGURE 46 REVENUE ANALYSIS OF KEY PLAYERS IN MOLECULAR CYTOGENETICS MARKET, 2020-2024 (USD MILLION)
- FIGURE 47 MARKET SHARE ANALYSIS OF KEY PLAYERS IN MOLECULAR CYTOGENETICS MARKET (2024)
- FIGURE 48 EV/EBITDA OF KEY VENDORS
- FIGURE 49 YEAR-TO-DATE (YTD) PRICE, TOTAL RETURN, AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 50 MOLECULAR CYTOGENETICS MARKET: BRAND/PRODUCT COMPARATIVE ANALYSIS
- FIGURE 51 MOLECULAR CYTOGENETICS MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 52 MOLECULAR CYTOGENETICS MARKET: COMPANY FOOTPRINT
- FIGURE 53 MOLECULAR CYTOGENETICS MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 54 F. HOFFMANN-LA ROCHE LTD.: COMPANY SNAPSHOT
- FIGURE 55 DANAHER CORPORATION: COMPANY SNAPSHOT
- FIGURE 56 AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT
- FIGURE 57 ABBOTT: COMPANY SNAPSHOT
- FIGURE 58 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT
- FIGURE 59 ILLUMINA, INC.: COMPANY SNAPSHOT
- FIGURE 60 REVVITY: COMPANY SNAPSHOT
- FIGURE 61 PACBIO: COMPANY SNAPSHOT
- FIGURE 62 BIO-RAD LABORATORIES, INC.: COMPANY SNAPSHOT
- FIGURE 63 BIO-TECHNE: COMPANY SNAPSHOT
- FIGURE 64 GENE DX, LLC: COMPANY SNAPSHOT
- FIGURE 65 INSIGHT MOLECULAR DIAGNOSTICS INC.: COMPANY SNAPSHOT











