 |
市場調查報告書
商品編碼
1861052
全球動物生技藥品市場按產品類型、動物類型、給藥途徑、應用、最終用戶和地區分類-預測至2030年Veterinary Biologics Market by Product (Monoclonal Antibodies, Diagnostic Kits, Immunoglobulins & Antitoxins), RoA (Injectable, Oral), Application (Infectious Disease Prevention, Dermatology, Pain Management), Animal, End User - Global Forecast to 2030 |
||||||
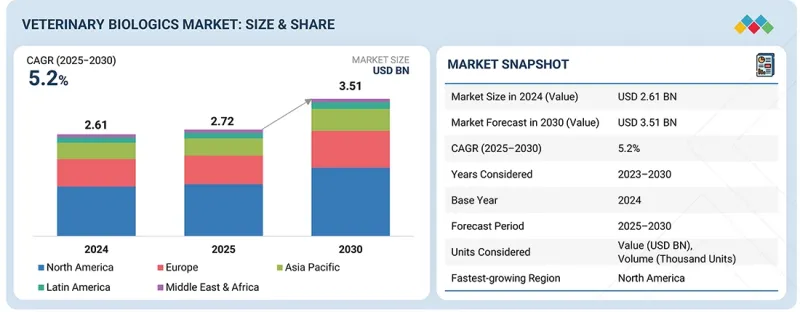
全球獸用生技藥品市場預計將從 2025 年的 27.2 億美元成長到 2030 年的 35.1 億美元,預測期內複合年成長率為 5.2%。
| 調查範圍 | |
|---|---|
| 調查年度 | 2024-2033 |
| 基準年 | 2024 |
| 預測期 | 2025-2030 |
| 考慮單位 | 金額(十億美元) |
| 部分 | 按產品類型、動物類型、給藥途徑、應用、最終用戶和地區 |
| 目標區域 | 北美、歐洲、亞太地區、拉丁美洲、中東和非洲、海灣合作理事會國家 |
受伴侶動物數量成長和寵物飼養率上升的推動,獸用生技藥品市場正經歷顯著成長。人們對慢性疾病日益關注,也進一步推動了對獸醫治療的需求。各國政府和動物福利組織正積極進行宣導宣傳活動,鼓勵對寵物和牲畜的皮膚病進行早期診斷和治療。
此外,寵物保險的普及和寵物醫療保健的高支出,透過提高寵物獲得專業獸醫服務的途徑,促進了市場擴張。然而,挑戰依然存在,例如寵物醫療成本不斷上漲,阻礙了治療的普及。此外,嚴格的監管準則和冗長的醫藥品認證過程也進一步限制了市場成長。
由於單株抗體具有標靶作用、高效性以及在畜牧業健康管理中日益廣泛的應用,預計其在獸用生技藥品市場將迎來快速成長。這些生技藥品透過精準靶向病原體和毒素,擴大被用於預防和治療特定疾病,為抗生素等傳統療法提供了更具選擇性和更有效的替代方案。在畜牧業應用中,單株抗體尤其適用於需要快速免疫支持的疾病,從而降低發病率並提高生產力。單株抗體的使用符合全球向永續畜牧業發展和降低抗菌素抗藥性(AMR)的趨勢,使其成為先進生物療法的首選。
由於伴侶動物中異位性皮膚炎、疥瘡和蜱蟲傳播的皮膚感染疾病等皮膚疾病的高發,預計皮膚病領域將主導獸用生技藥品市場。寵物主人數量的增加和人們對動物健康意識的提高,推動了對先進皮膚病治療的需求。生技藥品,例如單株抗體,因其標靶治療顯著且副作用少於傳統藥物,正日益受到歡迎。越來越多的皮膚病專用獸用生技藥品核准,包括針對慢性皮膚過敏的藥物,進一步鞏固了這個領域。此外,皮膚病是寵物就診的最常見原因之一,確保了穩定的需求。伴侶動物保險覆蓋範圍的擴大也推動了高成本生技藥品的使用。總而言之,這些因素使皮膚病成為獸用生技藥品市場的關鍵細分領域。
北美在2024年佔據了最大的市場佔有率,這得益於其完善的獸醫醫療保健基礎設施和較高的寵物擁有率。該地區聚集了許多市場主要相關人員,他們正大力投資於創新單株抗體療法的研發。寵物健康意識的提高和獸醫支出的增加正在推動市場成長。此外,對高階寵物護理產品需求的成長也鞏固了北美在全球獸用單株抗體產業的領先地位。
本報告對全球動物生技藥品市場進行了分析,並按產品類型、動物類型、給藥途徑、應用、最終用戶、區域趨勢以及參與市場的公司的概況進行了細分。
目錄
第1章 引言
第2章調查方法
第3章執行摘要
第4章重要考察
第5章 市場概覽
- 介紹
- 市場動態
- 技術分析
- 影響客戶業務的趨勢/顛覆性因素
- 價值鏈分析
- 貿易分析
- 波特五力分析
- 主要相關人員和採購標準
- 監管分析
- 專利分析
- 定價分析
- 2025-2026 年主要會議和活動
- 生態系分析
- 報銷分析
- 未滿足的需求
- 人工智慧/生成式人工智慧對動物生技藥品市場的影響
- 投資和資金籌措方案
- 管道分析
- 2025年美國關稅對動物生技藥品市場的影響
6. 按產品類型分類的生技藥品市場
- 介紹
- 單株抗體
- 免疫球蛋白和抗毒素
- 免疫調節劑和免疫促效劑
- 診斷試劑套件
7. 依動物類型分類的動物生技藥品市場
- 介紹
- 伴侶動物
- 家畜
第8章:動物生技藥品市場(依給藥途徑)
- 介紹
- 注射途徑
- 口服
- 其他
第9章:按應用分類的動物生技藥品市場
- 介紹
- 皮膚科
- 診斷測試
- 疼痛管理
- 感染疾病預防
- 腫瘤學
- 其他
第10章 動物生技藥品市場(依最終用戶分類)
- 介紹
- 獸醫醫院和診所
- 獸醫診斷實驗室
- 其他
第11章:按地區分類的動物生技藥品市場
- 介紹
- 北美洲
- 北美宏觀經濟展望
- 美國
- 加拿大
- 歐洲
- 歐洲宏觀經濟展望
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 荷蘭
- 其他
- 亞太地區
- 亞太宏觀經濟展望
- 日本
- 中國
- 印度
- 澳洲
- 韓國
- 紐西蘭
- 其他
- 拉丁美洲
- 拉丁美洲宏觀經濟展望
- 巴西
- 墨西哥
- 其他
- 中東和非洲
- 中東和非洲宏觀經濟展望
- 海灣合作理事會國家
- 其他
第12章 競爭格局
- 介紹
- 主要參與企業的策略/優勢
- 2022-2024年收入分析
- 2024年市佔率分析
- 主要參與企業排名
- 公司估值矩陣:主要參與企業,2024 年
- 公司估值矩陣:Start-Ups/中小企業,2023 年
- 品牌/產品對比
- 估值和財務指標
- 競爭場景
第13章 公司概況
- 主要參與企業
- ZOETIS SERVICES LLC
- IDEXX
- ELANCO
- MERCK & CO., INC.
- THERMO FISHER SCIENTIFIC INC.
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH
- NEOGEN CORPORATION
- BIO-RAD LABORATORIES, INC.
- IMMUCELL CORPORATION
- INNOVATIVE DIAGNOSTICS
- 其他公司
- BIONOTE USA INC.
- BIOCHEK
- PLASVACC USA
- NOVAVIVE INC.
- MG BIOLOGICS
- STALLERGENES GREER
- BIOSTONE ANIMAL HEALTH
- COLORADO SERUM COMPANY
- PADULA SERUMS PTY LTD.
- LAKE IMMUNOGENICS
- INBIOS INTERNATIONAL, INC.
- PRINCETON BIOMEDITECH CORPORATION
- TETRACORE, INC.
- VMRD, INC.
- ANIMAB
第14章附錄
The global veterinary biologics market is projected to reach USD 3.51 billion by 2030 from USD 2.72 billion in 2025, at a CAGR of 5.2% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2033 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Product type, Animal Type, Route of Administration, Application, End user, Region |
| Regions covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa, and GCC Countries |
The veterinary biologics market is experiencing significant growth, driven by an expanding companion animal population and rising pet ownership. Increasing concerns about chronic diseases have further boosted demand for veterinary treatments. Government and animal welfare organizations actively promote awareness campaigns, encouraging early diagnosis and treatment of dermatological conditions in both pets and farm animals.

Additionally, the growing adoption of pet insurance and high expenditure on animal healthcare support market expansion by improving access to specialized veterinary services. However, the market faces challenges such as the rising cost of pet care, which can limit treatment adoption. Moreover, strict regulatory guidelines and lengthy drug approval processes further restrain market growth.
"The monoclonal antibodies segment has accounted for the largest market share in the veterinary biologics market."
Monoclonal antibodies are projected to experience rapid growth in the veterinary biologics market, driven by their targeted action, high effectiveness, and increasing adoption in livestock health management. These biologics are increasingly used to prevent and treat specific diseases by precisely targeting pathogens or toxins, providing a more selective and effective alternative to traditional therapies like antibiotics. In livestock applications, monoclonal antibodies are especially valuable for conditions that require swift immune support, resulting in decreased morbidity and higher productivity. Their use aligns with the global shift toward sustainable animal farming practices and the reduction of antimicrobial resistance (AMR), making them a preferred choice for advanced biologic treatments.
"The dermatology segment accounted for the largest market share in 2024 in the veterinary biologics market."
The dermatology segment is expected to dominate the veterinary biologics market because of the high prevalence of skin disorders like atopic dermatitis, mange, and flea- or tick-borne skin infections in companion animals. Increasing pet ownership and greater awareness of animal health have boosted demand for advanced dermatological treatments. Biologics, such as monoclonal antibodies, are becoming more popular as effective, targeted therapies with fewer side effects compared to traditional drugs. More approvals of dermatology-focused veterinary biologics, including those targeting chronic skin allergies, further strengthen this segment. Additionally, dermatological issues are among the most common reasons for veterinary visits, ensuring steady demand. Growth in companion animal insurance coverage is also supporting the adoption of high-cost biologics. Overall, these factors position dermatology as the leading segment in the veterinary biologics market.
"North America has accounted for the largest market share in 2024."
North America held the largest market share in 2024, supported by a well-established veterinary healthcare infrastructure and high pet ownership rates. The region has a strong presence of leading market players investing in research and development for innovative monoclonal antibody therapies. Rising awareness about pet health, along with increasing spending on veterinary care, drives market growth. Additionally, the growing demand for premium pet care products further contributes to North America's dominance in the global veterinary monoclonal antibody industry.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1-75%, Tier 2-15%, and Tier 3- 10%
- By Designation: C-level-30%, Director-level-23%, and Other Designations-47%
- By Region: North America-35%, Europe-20%, Asia Pacific-25%, Latin America-13%, and Middle East & Africa-7%
The major players operating in the veterinary biologics market are Zoetis (US), Elanco (US), and Merck & Co., Inc. (US).
Research Coverage
This report studies the veterinary biologics market based on animal type, route of administration, product, application, end user, and region. The report also examines factors such as drivers, restraints, opportunities, and challenges that affect market growth. It analyzes the opportunities and challenges within the market and provides details about the competitive landscape for market leaders. Additionally, the report analyzes micro markets in terms of their individual growth trends and forecasts the revenue of market segments across five main regions and their respective countries.
Reasons to Buy the Report
The report can help established firms, as well as new entrants/smaller firms, gauge the pulse of the market, which, in turn, would help them garner a greater share. Firms purchasing the report could use one or a combination of the five strategies mentioned below.
This report provides insights into the following points:
- Analysis of key drivers (Surge in companion animal population and pet ownership. Increasing incidence of infectious zoonotic diseases, Innovations in monoclonal antibodies targeting infectious diseases, Supportive initiatives by government agencies and animal health organization, Growing demand for animal-derived food products), restraints (High cost of veterinary diagnostic tests, Regulatory hurdles and long approval timelines), opportunities (evolving landscape of strategic collaborations and partnerships, untapped growth potential in emerging markets, challenges (Limited Awareness and Shortage of Trained Veterinarians in Low-Income Countries.
- Product Development/Innovation: Detailed insights on upcoming technologies, research and development activities, and product launches in the veterinary biologics market.
- Market Development: Comprehensive information about lucrative emerging markets. The report analyzes the markets for various types of biologic treatments across regions.
- Market Diversification: Exhaustive information about products, untapped regions, recent developments, and investments in the veterinary biologics market.
- Competitive Assessment: In-depth assessment of market shares, strategies, products, distribution networks, and manufacturing capabilities of the leading players in the veterinary biologics market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key secondary sources
- 2.1.1.2 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Primary sources
- 2.1.2.2 Key objectives of primary research
- 2.1.2.3 Key industry insights
- 2.1.2.4 Breakdown of primaries
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 REVENUE SHARE ANALYSIS (BOTTOM-UP APPROACH)
- 2.2.2 EPIDEMIOLOGY-BASED APPROACH
- 2.2.3 COMPANY INVESTOR PRESENTATIONS AND PRIMARY INTERVIEWS
- 2.2.4 TOP-DOWN APPROACH
- 2.2.5 BOTTOM-UP APPROACH
- 2.3 MARKET GROWTH RATE PROJECTION
- 2.4 DATA TRIANGULATION
- 2.5 STUDY ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 VETERINARY BIOLOGICS MARKET OVERVIEW
- 4.2 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE AND COUNTRY
- 4.3 VETERINARY BIOLOGICS MARKET SHARE, BY PRODUCT TYPE, 2025 VS. 2030
- 4.4 VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2025 VS. 2030 (USD MILLION)
- 4.5 VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2025 VS. 2030 (USD MILLION)
- 4.6 VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- 4.7 VETERINARY BIOLOGICS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Surge in companion animal population and pet ownership
- 5.2.1.2 Increasing incidence of infectious zoonotic diseases
- 5.2.1.3 Innovations in monoclonal antibodies targeting infectious diseases
- 5.2.1.4 Supportive initiatives by government agencies and animal health organizations
- 5.2.1.5 Rising demand for animal-derived food products
- 5.2.2 RESTRAINTS
- 5.2.2.1 High cost of veterinary diagnostic tests
- 5.2.2.2 Regulatory hurdles and long approval timelines
- 5.2.2.3 Rising pet care costs
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Evolving landscape of strategic collaborations and acquisitions
- 5.2.3.2 Untapped growth potential in emerging economies
- 5.2.4 CHALLENGES
- 5.2.4.1 Limited awareness and shortage of trained veterinarians in low-income countries
- 5.2.4.2 Stringent regulatory requirements for licensing veterinary biologics
- 5.2.1 DRIVERS
- 5.3 TECHNOLOGY ANALYSIS
- 5.3.1 KEY TECHNOLOGIES
- 5.3.1.1 Hybridoma technology
- 5.3.1.2 Biosensor-based diagnostic systems
- 5.3.2 COMPLEMENTARY TECHNOLOGIES
- 5.3.2.1 Long-acting injectables
- 5.3.2.2 Next-generation sequencing
- 5.3.3 ADJACENT TECHNOLOGIES
- 5.3.3.1 Wearable biosensors & remote monitoring devices
- 5.3.1 KEY TECHNOLOGIES
- 5.4 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 TRADE ANALYSIS
- 5.6.1 IMPORT DATA FOR HS CODE 300214, 2021-2024
- 5.6.2 EXPORT DATA FOR HS CODE 300214, 2021-2024
- 5.7 PORTER'S FIVE FORCES ANALYSIS
- 5.7.1 THREAT OF NEW ENTRANTS
- 5.7.2 THREAT OF SUBSTITUTES
- 5.7.3 BARGAINING POWER OF SUPPLIERS
- 5.7.4 BARGAINING POWER OF BUYERS
- 5.7.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.8 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.8.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.8.2 KEY BUYING CRITERIA
- 5.9 REGULATORY ANALYSIS
- 5.9.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.9.2 REGULATORY FRAMEWORK
- 5.9.2.1 North America
- 5.9.2.2 Europe
- 5.9.2.3 Asia Pacific
- 5.9.2.4 Latin America
- 5.9.2.5 Middle East & Africa
- 5.10 PATENT ANALYSIS
- 5.10.1 PATENT PUBLICATION TREND
- 5.10.2 JURISDICTION AND TOP APPLICANT ANALYSIS
- 5.10.3 INNOVATIONS AND PATENT REGISTRATIONS
- 5.11 PRICING ANALYSIS
- 5.11.1 INDICATIVE PRICE OF VETERINARY MONOCLONAL ANTIBODIES, BY KEY PLAYER, 2024
- 5.11.2 INDICATIVE PRICE OF VETERINARY MONOCLONAL ANTIBODIES, BY REGION, 2022-2024
- 5.12 KEY CONFERENCES & EVENTS, 2025-2026
- 5.13 ECOSYSTEM ANALYSIS
- 5.13.1 ROLE IN ECOSYSTEM
- 5.14 REIMBURSEMENT ANALYSIS
- 5.15 UNMET NEEDS
- 5.16 IMPACT OF AI/GEN AI ON VETERINARY BIOLOGICS MARKET
- 5.16.1 POTENTIAL OF AI IN VETERINARY BIOLOGICS
- 5.16.2 AI USE CASES
- 5.16.3 KEY COMPANIES IMPLEMENTING AI
- 5.17 INVESTMENT & FUNDING SCENARIO
- 5.18 PIPELINE ANALYSIS
- 5.19 IMPACT OF 2025 US TARIFF ON VETERINARY BIOLOGICS MARKET
- 5.19.1 INTRODUCTION
- 5.19.2 KEY TARIFF RATES
- 5.19.3 PRICE IMPACT ANALYSIS
- 5.19.4 IMPACT ON COUNTRY/REGION
- 5.19.5 IMPACT ON END-USE INDUSTRIES
- 5.19.5.1 Veterinary hospitals & clinics
- 5.19.5.2 Veterinary diagnostic labs
- 5.19.5.3 Other end users
6 VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE
- 6.1 INTRODUCTION
- 6.2 MONOCLONAL ANTIBODIES
- 6.2.1 INCREASED PET OWNERSHIP AND HUMANIZATION TO RAISE HEALTH AWARENESS AND SPENDING ON ANTIBODY THERAPIES
- 6.3 IMMUNOGLOBULINS & ANTITOXINS
- 6.3.1 INCREASED PREVALENCE OF ZOONOTIC AND TOXIN-MEDIATED DISEASES TO FUEL MARKET GROWTH
- 6.4 IMMUNOMODULATORS & IMMUNOSTIMULANTS
- 6.4.1 IMMUNOMODULATORS & IMMUNOSTIMULANTS TO CORRECT IMMUNE DYSFUNCTIONS AND CONTROL OVERACTIVE IMMUNE CONDITIONS
- 6.5 DIAGNOSTIC KITS
- 6.5.1 DIAGNOSTIC KITS TO IMPROVE DISEASE SURVEILLANCE AND MANAGEMENT IN COMPANION ANIMALS, LIVESTOCK, AND POULTRY
7 VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE
- 7.1 INTRODUCTION
- 7.2 COMPANION ANIMALS
- 7.2.1 CANINE
- 7.2.1.1 Widespread ownership of pet dogs to generate consistent demand for preventive care and advanced biologics
- 7.2.2 FELINE
- 7.2.2.1 Rising pet cat population and increasing feline diseases to propel market growth
- 7.2.1 CANINE
- 7.3 LIVESTOCK ANIMALS
- 7.3.1 BOVINE
- 7.3.1.1 Need for better herd healthcare and introduction of antibody-based biologics to fuel segment growth
- 7.3.2 SMALL RUMINANT
- 7.3.2.1 Increasing global demand for sheep & goat meat and expanding herd size to boost market growth
- 7.3.3 PORCINE
- 7.3.3.1 Innovation in monoclonal antibodies for infectious diseases to drive market
- 7.3.4 POULTRY
- 7.3.4.1 Rising frequency of poultry diseases to augment market growth for better poultry health and improved food security
- 7.3.5 EQUINE
- 7.3.5.1 Equestrian sports, recreational riding, and managing failure of passive transfer (FPT) in foals to drive market
- 7.3.1 BOVINE
8 VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION
- 8.1 INTRODUCTION
- 8.2 INJECTABLE ROUTE OF ADMINISTRATION
- 8.2.1 SUBCUTANEOUS ROUTE OF ADMINISTRATION
- 8.2.1.1 Ease of administration, reduced pain, and lower risk of tissue damage to aid market growth
- 8.2.2 INTRAMUSCULAR ROUTE OF ADMINISTRATION
- 8.2.2.1 Better immune response and lower rate of injection-site reactions to fuel market growth
- 8.2.3 INTRAVENOUS ROUTE OF ADMINISTRATION
- 8.2.3.1 Rapid action and targeted therapy to drive adoption of intravenous biologics
- 8.2.4 OTHER INJECTABLE ROUTES OF ADMINISTRATION
- 8.2.1 SUBCUTANEOUS ROUTE OF ADMINISTRATION
- 8.3 ORAL ROUTE OF ADMINISTRATION
- 8.3.1 EASE OF ADMINISTRATION AND COST-EFFECTIVENESS TO FOCUS ON LARGE-SCALE, NON-INVASIVE ORAL DELIVERY
- 8.4 OTHER ROUTES OF ADMINISTRATION
9 VETERINARY BIOLOGICS MARKET, BY APPLICATION
- 9.1 INTRODUCTION
- 9.2 DERMATOLOGY
- 9.2.1 PREVALENCE OF CHRONIC SKIN CONDITIONS TO AUGMENT VETERINARY DERMATOLOGY MARKET GROWTH
- 9.3 DIAGNOSTIC TESTING
- 9.3.1 RISING PREVALENCE OF ZOONOTIC AND VIRAL DISEASES TO INCREASE DEMAND FOR BIOLOGIC-BASED DIAGNOSTIC TOOLS
- 9.4 PAIN MANAGEMENT
- 9.4.1 NEED FOR NON-OPIOID, SIDE-EFFECT-FREE PAIN SOLUTIONS AMONG PET OWNERS TO DRIVE MARKET
- 9.5 INFECTIOUS DISEASE PREVENTION
- 9.5.1 RISING INCIDENCE OF ZOONOTIC DISEASES AND ACUTE INFECTIONS TO ACCELERATE USE OF VETERINARY BIOLOGICS
- 9.6 ONCOLOGY
- 9.6.1 FOCUS ON IMMUNE-TARGETED CONTROL OF AGGRESSIVE CANCEROUS TUMORS TO AID MARKET GROWTH
- 9.7 OTHER APPLICATIONS
10 VETERINARY BIOLOGICS MARKET, BY END USER
- 10.1 INTRODUCTION
- 10.2 VETERINARY HOSPITALS & CLINICS
- 10.2.1 INCREASE IN VETERINARY SPECIALISTS AND RISE IN PET OWNERSHIP TO PROPEL MARKET GROWTH
- 10.3 VETERINARY DIAGNOSTIC LABS
- 10.3.1 VETERINARY DIAGNOSTIC LABS TO BECOME ESSENTIAL IN DISEASE DETECTION WITH PREVENTIVE BIOLOGIC INTERVENTIONS
- 10.4 OTHER END USERS
11 VETERINARY BIOLOGICS MARKET, BY REGION
- 11.1 INTRODUCTION
- 11.2 NORTH AMERICA
- 11.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 11.2.2 US
- 11.2.2.1 US to dominate North American veterinary biologics market during forecast period
- 11.2.3 CANADA
- 11.2.3.1 Ongoing developments in veterinary monoclonal antibodies and increasing animal health expenditure to fuel market growth
- 11.3 EUROPE
- 11.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 11.3.2 GERMANY
- 11.3.2.1 Established veterinary infrastructure and innovation funding to provide favorable environment for biologics adoption
- 11.3.3 UK
- 11.3.3.1 Strong regulatory framework and high pet population to boost market growth
- 11.3.4 FRANCE
- 11.3.4.1 Increase in companion animal health spending by pet owners to propel market growth
- 11.3.5 ITALY
- 11.3.5.1 Better availability of pet products through large-scale outlets to augment market growth
- 11.3.6 SPAIN
- 11.3.6.1 Increased pet population and favorable government regulations to drive market
- 11.3.7 NETHERLANDS
- 11.3.7.1 Focus on meat export to positively influence veterinary biologics market
- 11.3.8 REST OF EUROPE
- 11.4 ASIA PACIFIC
- 11.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 11.4.2 JAPAN
- 11.4.2.1 Growing consumption of animal-derived products to propel market growth
- 11.4.3 CHINA
- 11.4.3.1 Large pool of food-producing animals and high companion animal ownership to propel market growth
- 11.4.4 INDIA
- 11.4.4.1 Rising demand for dairy products to fuel market growth
- 11.4.5 AUSTRALIA
- 11.4.5.1 Rise in pet ownership to support increased spending on veterinary care
- 11.4.6 SOUTH KOREA
- 11.4.6.1 Expanding pet care industry and increasing chronic veterinary diseases to drive market
- 11.4.7 NEW ZEALAND
- 11.4.7.1 Increased pet ownership and demand for advanced veterinary treatments to drive market
- 11.4.8 REST OF ASIA PACIFIC
- 11.5 LATIN AMERICA
- 11.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 11.5.2 BRAZIL
- 11.5.2.1 Large-scale livestock operations and strong veterinary infrastructure to drive market
- 11.5.3 MEXICO
- 11.5.3.1 Increasing demand for targeted biologics in livestock and pets to drive market growth
- 11.5.4 REST OF LATIN AMERICA
- 11.6 MIDDLE EAST & AFRICA
- 11.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
- 11.6.2 GCC COUNTRIES
- 11.6.2.1 Kingdom of Saudi Arabia (KSA)
- 11.6.2.1.1 Technology advancements in veterinary diagnostics to boost demand
- 11.6.2.2 United Arab Emirates (UAE)
- 11.6.2.2.1 Favorable government support to drive market
- 11.6.2.3 Rest of GCC Countries
- 11.6.2.1 Kingdom of Saudi Arabia (KSA)
- 11.6.3 REST OF MIDDLE EAST & AFRICA
12 COMPETITIVE LANDSCAPE
- 12.1 INTRODUCTION
- 12.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 12.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN VETERINARY BIOLOGICS MARKET
- 12.3 REVENUE ANALYSIS, 2022-2024
- 12.4 MARKET SHARE ANALYSIS, 2024
- 12.4.1 VETERINARY BIOLOGICS MARKET SHARE ANALYSIS
- 12.4.2 US: VETERINARY BIOLOGICS MARKET SHARE ANALYSIS, 2024
- 12.4.3 VETERINARY DIAGNOSTICS KITS MARKET SHARE ANALYSIS
- 12.4.4 US: VETERINARY DIAGNOSTIC KITS MARKET SHARE ANALYSIS, 2024
- 12.5 RANKING OF KEY PLAYERS
- 12.5.1 VETERINARY BIOLOGICS MARKET RANKING
- 12.5.2 VETERINARY DIAGNOSTIC KITS MARKET RANKING
- 12.6 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 12.6.1 STARS
- 12.6.2 EMERGING LEADERS
- 12.6.3 PERVASIVE PLAYERS
- 12.6.4 PARTICIPANTS
- 12.6.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 12.6.5.1 Company footprint
- 12.6.5.2 Region footprint
- 12.6.5.3 Product type footprint
- 12.6.5.4 Animal type footprint
- 12.6.5.5 Application footprint
- 12.7 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023
- 12.7.1 PROGRESSIVE COMPANIES
- 12.7.2 RESPONSIVE COMPANIES
- 12.7.3 DYNAMIC COMPANIES
- 12.7.4 STARTING BLOCKS
- 12.7.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 12.7.5.1 Detailed list of key startup/SMEs
- 12.7.5.2 Competitive benchmarking of key startups/SMEs
- 12.8 BRAND/PRODUCT COMPARISON
- 12.9 COMPANY VALUATION & FINANCIAL METRICS
- 12.9.1 FINANCIAL METRICS
- 12.9.2 COMPANY VALUATION
- 12.10 COMPETITIVE SCENARIO
- 12.10.1 PRODUCT LAUNCHES & APPROVALS
- 12.10.2 DEALS
- 12.10.3 EXPANSIONS
- 12.10.4 OTHER DEVELOPMENTS
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- 13.1.1 ZOETIS SERVICES LLC
- 13.1.1.1 Business overview
- 13.1.1.2 Products offered
- 13.1.1.3 Recent developments
- 13.1.1.3.1 Product launches & approvals
- 13.1.1.3.2 Deals
- 13.1.1.3.3 Expansions
- 13.1.1.3.4 Other developments
- 13.1.1.4 MnM view
- 13.1.1.4.1 Right to win
- 13.1.1.4.2 Strategic choices
- 13.1.1.4.3 Weaknesses & competitive threats
- 13.1.2 IDEXX
- 13.1.2.1 Business overview
- 13.1.2.2 Products offered
- 13.1.2.3 Recent developments
- 13.1.2.3.1 Product launches
- 13.1.2.4 MnM view
- 13.1.2.4.1 Right to win
- 13.1.2.4.2 Strategic choices
- 13.1.2.4.3 Weaknesses & competitive threats
- 13.1.3 ELANCO
- 13.1.3.1 Business overview
- 13.1.3.2 Products offered
- 13.1.3.3 Recent developments
- 13.1.3.3.1 Product launches
- 13.1.3.3.2 Deals
- 13.1.3.3.3 Expansions
- 13.1.3.4 MnM view
- 13.1.3.4.1 Right to win
- 13.1.3.4.2 Strategic choices
- 13.1.3.4.3 Weaknesses & competitive threats
- 13.1.4 MERCK & CO., INC.
- 13.1.4.1 Business overview
- 13.1.4.2 Products offered
- 13.1.4.3 Recent developments
- 13.1.4.3.1 Product launches
- 13.1.4.3.2 Expansions
- 13.1.4.4 MnM view
- 13.1.4.4.1 Right to win
- 13.1.4.4.2 Strategic choices
- 13.1.4.4.3 Weaknesses & competitive threats
- 13.1.5 THERMO FISHER SCIENTIFIC INC.
- 13.1.5.1 Business overview
- 13.1.5.2 Products offered
- 13.1.5.3 MnM view
- 13.1.5.3.1 Right to win
- 13.1.5.3.2 Strategic choices
- 13.1.5.3.3 Weaknesses & competitive threats
- 13.1.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
- 13.1.6.1 Business overview
- 13.1.6.2 Products offered
- 13.1.6.3 Recent developments
- 13.1.6.3.1 Deals
- 13.1.6.3.2 Expansions
- 13.1.7 NEOGEN CORPORATION
- 13.1.7.1 Business overview
- 13.1.7.2 Products offered
- 13.1.7.3 Recent developments
- 13.1.7.3.1 Deals
- 13.1.8 BIO-RAD LABORATORIES, INC.
- 13.1.8.1 Business overview
- 13.1.8.2 Products offered
- 13.1.9 IMMUCELL CORPORATION
- 13.1.9.1 Business overview
- 13.1.9.2 Products offered
- 13.1.9.3 Recent developments
- 13.1.9.3.1 Other developments
- 13.1.10 INNOVATIVE DIAGNOSTICS
- 13.1.10.1 Business overview
- 13.1.10.2 Products offered
- 13.1.1 ZOETIS SERVICES LLC
- 13.2 OTHER PLAYERS
- 13.2.1 BIONOTE USA INC.
- 13.2.2 BIOCHEK
- 13.2.3 PLASVACC USA
- 13.2.4 NOVAVIVE INC.
- 13.2.5 MG BIOLOGICS
- 13.2.6 STALLERGENES GREER
- 13.2.7 BIOSTONE ANIMAL HEALTH
- 13.2.8 COLORADO SERUM COMPANY
- 13.2.9 PADULA SERUMS PTY LTD.
- 13.2.10 LAKE IMMUNOGENICS
- 13.2.11 INBIOS INTERNATIONAL, INC.
- 13.2.12 PRINCETON BIOMEDITECH CORPORATION
- 13.2.13 TETRACORE, INC.
- 13.2.14 VMRD, INC.
- 13.2.15 ANIMAB
14 APPENDIX
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.3 AVAILABLE CUSTOMIZATIONS
- 14.3.1 GEOGRAPHIC ANALYSIS
- 14.3.2 REGIONAL/COUNTRY-LEVEL MARKET SHARE ANALYSIS
- 14.3.3 COMPANY INFORMATION
- 14.3.4 PRODUCT ANALYSIS
- 14.3.5 COUNTRY-LEVEL VOLUME ANALYSIS
- 14.3.6 ANY CONSULTS/CUSTOM REQUIREMENTS AS PER CLIENT REQUEST
- 14.3.7 BY PRODUCT TYPE MARKET SHARE ANALYSIS (TOP 5 PLAYERS)
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS
List of Tables
- TABLE 1 VETERINARY BIOLOGICS MARKET: INCLUSIONS & EXCLUSIONS
- TABLE 2 VETERINARY BIOLOGICS MARKET: STUDY ASSUMPTIONS
- TABLE 3 VETERINARY BIOLOGICS MARKET: RISK ANALYSIS
- TABLE 4 PET (DOGS AND CATS) OWNERSHIP AND SPENDING IN US, 2024
- TABLE 5 MAJOR OUTBREAKS OF ZOONOTIC DISEASES, 2022-2025
- TABLE 6 PAST AND PROJECTED TRENDS IN MEAT AND MILK CONSUMPTION IN DEVELOPED COUNTRIES, 2002 VS. 2015 VS. 2030 VS. 2050
- TABLE 7 ASIA: COUNTRY-LEVEL CONSUMPTION OF ANIMAL-DERIVED FOOD PRODUCTS, 2020 VS. 2030 (THOUSAND METRIC TONS)
- TABLE 8 ASIA: COUNTRY-LEVEL PRODUCTION OF ANIMAL-DERIVED FOOD PRODUCTS, 2020 VS. 2030 (THOUSAND METRIC TONS)
- TABLE 9 IMPORT DATA FOR HS CODE 300214, BY COUNTRY, 2021-2024 (USD THOUSAND)
- TABLE 10 EXPORT DATA FOR HS CODE 300214, BY COUNTRY, 2021-2024 (USD THOUSAND)
- TABLE 11 VETERINARY BIOLOGICS MARKET: PORTER'S FIVE FORCES
- TABLE 12 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS OF VETERINARY BIOLOGICS, BY END USER
- TABLE 13 KEY BUYING CRITERIA FOR END USERS
- TABLE 14 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 15 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 16 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 17 VETERINARY BIOLOGICS MARKET: INNOVATIONS AND PATENT REGISTRATIONS OF VETERINARY MONOCLONAL ANTIBODIES, 2023-2024
- TABLE 18 INDICATIVE PRICE OF VETERINARY MONOCLONAL ANTIBODIES, BY KEY PLAYER, 2024 (USD)
- TABLE 19 INDICATIVE SELLING PRICE OF VETERINARY MONOCLONAL ANTIBODIES, BY REGION, 2022-2024 (USD)
- TABLE 20 DETAILED LIST OF KEY CONFERENCES & EVENTS IN VETERINARY BIOLOGICS MARKET, JANUARY 2025-DECEMBER 2026
- TABLE 21 VETERINARY BIOLOGICS MARKET: ROLE IN ECOSYSTEM
- TABLE 22 UNMET NEEDS IN VETERINARY BIOLOGICS MARKET
- TABLE 23 KEY COMPANIES IMPLEMENTING AI IN VETERINARY BIOLOGICS MARKET
- TABLE 24 PIPELINE ANALYSIS IN VETERINARY BIOLOGICS MARKET
- TABLE 25 US-ADJUSTED RECIPROCAL TARIFF RATES
- TABLE 26 KEY PRODUCT-RELATED TARIFF EFFECTIVE FOR VETERINARY BIOLOGICS MARKET
- TABLE 27 IMPACT OF US TARIFFS ON COUNTRIES/REGIONS
- TABLE 28 VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 29 LIST OF COMMERCIALLY AVAILABLE MONOCLONAL ANTIBODIES
- TABLE 30 VETERINARY BIOLOGICS MARKET FOR MONOCLONAL ANTIBODIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 31 LIST OF USDA-APPROVED IMMUNOGLOBULINS & ANTITOXINS
- TABLE 32 VETERINARY BIOLOGICS MARKET FOR IMMUNOGLOBULINS & ANTITOXINS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 33 VETERINARY BIOLOGICS MARKET FOR IMMUNOMODULATORS & IMMUNOSTIMULANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 34 LIST OF LICENSED VETERINARY DIAGNOSTIC KITS AS APPROVED BY US FDA CENTER FOR VETERINARY BIOLOGICS
- TABLE 35 VETERINARY BIOLOGICS MARKET FOR DIAGNOSTIC KITS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 36 VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 37 COMPANION ANIMALS OWNERSHIP IN US, 2024
- TABLE 38 VETERINARY BIOLOGICS MARKET FOR COMPANION ANIMALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 39 VETERINARY BIOLOGICS MARKET FOR COMPANION ANIMALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 40 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR CANINES
- TABLE 41 VETERINARY BIOLOGICS MARKET FOR CANINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 42 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR FELINES
- TABLE 43 VETERINARY BIOLOGICS MARKET FOR FELINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 44 VETERINARY BIOLOGICS MARKET FOR LIVESTOCK ANIMALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 45 VETERINARY BIOLOGICS MARKET FOR LIVESTOCK ANIMALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 46 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR BOVINES
- TABLE 47 VETERINARY BIOLOGICS MARKET FOR BOVINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 48 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR SMALL RUMINANTS
- TABLE 49 VETERINARY BIOLOGICS MARKET FOR SMALL RUMINANT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 50 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR PORCINES
- TABLE 51 VETERINARY BIOLOGICS MARKET FOR PORCINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 52 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR POULTRY
- TABLE 53 VETERINARY BIOLOGICS MARKET FOR POULTRY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 54 KEY PLAYERS PROVIDING VETERINARY BIOLOGICS FOR EQUINES
- TABLE 55 VETERINARY BIOLOGICS MARKET FOR EQUINE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 56 VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 57 VETERINARY BIOLOGICS MARKET FOR INJECTABLE ROUTE OF ADMINISTRATION, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 58 VETERINARY BIOLOGICS MARKET FOR INJECTABLE ROUTE OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 59 VETERINARY BIOLOGICS MARKET FOR SUBCUTANEOUS ROUTE OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 60 VETERINARY BIOLOGICS MARKET FOR INTRAMUSCULAR ROUTE OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 61 VETERINARY BIOLOGICS MARKET FOR INTRAVENOUS ROUTE OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 62 VETERINARY BIOLOGICS MARKET FOR OTHER INJECTABLE ROUTES OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 63 VETERINARY BIOLOGICS MARKET FOR ORAL ROUTE OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 64 VETERINARY BIOLOGICS MARKET FOR OTHER ROUTES OF ADMINISTRATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 65 VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 66 VETERINARY BIOLOGICS MARKET FOR DERMATOLOGY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 67 VETERINARY BIOLOGICS MARKET FOR DIAGNOSTIC TESTNG, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 68 VETERINARY BIOLOGICS MARKET FOR PAIN MANAGEMENT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 69 VETERINARY BIOLOGICS MARKET FOR INFECTIOUS DISEASE PREVENTION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 70 VETERINARY BIOLOGICS MARKET FOR ONCOLOGY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 71 VETERINARY BIOLOGICS MARKET FOR OTHER APPLICATIONS,BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 72 VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 73 AVERAGE TOTAL AMOUNT SPENT PER HOUSEHOLD ON PETS IN US, 2023-2024 (USD)
- TABLE 74 VETERINARY BIOLOGICS MARKET FOR VETERINARY HOSPITALS & CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 75 VETERINARY BIOLOGICS MARKET FOR VETERINARY DIAGNOSTIC LABS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 76 VETERINARY BIOLOGICS MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 77 VETERINARY BIOLOGICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 78 VETERINARY BIOLOGICS MARKET FOR MONOCLONAL ANTIBODIES, BY REGION, 2023-2030 (THOUSAND UNITS)
- TABLE 79 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY COUNTRY 2023-2030 (USD MILLION
- TABLE 80 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 81 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 82 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 83 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 84 NORTH AMERICA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 85 US: KEY MACROINDICATORS
- TABLE 86 US: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 87 US: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 88 US: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 89 US: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 90 US: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 91 CANADA: KEY MACROINDICATORS
- TABLE 92 CANADA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 93 CANADA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 94 CANADA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 95 CANADA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 96 CANADA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 97 EUROPE: VETERINARY BIOLOGICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION
- TABLE 98 EUROPE: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 99 EUROPE: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 100 EUROPE: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 101 EUROPE: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 102 EUROPE: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 103 GERMANY: KEY MACROINDICATORS
- TABLE 104 GERMANY: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 105 GERMANY: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 106 GERMANY: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 107 GERMANY: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 108 GERMANY: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 109 UK: KEY MACROINDICATORS
- TABLE 110 UK: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 111 UK: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 112 UK: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 113 UK: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 114 UK: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 115 FRANCE: KEY MACROINDICATORS
- TABLE 116 FRANCE: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 117 FRANCE: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 118 FRANCE: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 119 FRANCE: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 120 FRANCE: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 121 ITALY: KEY MACROINDICATORS
- TABLE 122 ITALY: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 123 ITALY: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 124 ITALY: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 125 ITALY: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 126 ITALY: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 127 SPAIN: KEY MACROINDICATORS
- TABLE 128 SPAIN: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 129 SPAIN: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 130 SPAIN: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 131 SPAIN: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 132 SPAIN: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 133 NETHERLANDS: KEY MACROINDICATORS
- TABLE 134 NETHERLANDS: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 135 NETHERLANDS: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 136 NETHERLANDS: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 137 NETHERLANDS: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 138 NETHERLANDS: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 139 REST OF EUROPE: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 140 REST OF EUROPE: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 141 REST OF EUROPE: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 142 REST OF EUROPE: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 143 REST OF EUROPE: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 144 ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY COUNTRY 2023-2030 (USD MILLION
- TABLE 145 ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 146 ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 147 ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 148 ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 149 ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 150 JAPAN: KEY MACROINDICATORS
- TABLE 151 JAPAN: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 152 JAPAN: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 153 JAPAN: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 154 JAPAN: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 155 JAPAN: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 156 CHINA: KEY MACROINDICATORS
- TABLE 157 CHINA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 158 CHINA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 159 CHINA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 160 CHINA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 161 CHINA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 162 INDIA: PRODUCTION AND CONSUMPTION OF ANIMAL-DERIVED FOOD PRODUCTS, 2000 VS. 2030 (THOUSAND METRIC TONS)
- TABLE 163 INDIA: KEY MACROINDICATORS
- TABLE 164 INDIA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 165 INDIA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 166 INDIA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 167 INDIA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 168 INDIA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 169 AUSTRALIA: KEY MACROINDICATORS
- TABLE 170 AUSTRALIA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 171 AUSTRALIA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 172 AUSTRALIA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 173 AUSTRALIA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 174 AUSTRALIA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 175 SOUTH KOREA: KEY MACROINDICATORS
- TABLE 176 SOUTH KOREA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 177 SOUTH KOREA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 178 SOUTH KOREA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 179 SOUTH KOREA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 180 SOUTH KOREA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 181 NEW ZEALAND: KEY MACROINDICATORS
- TABLE 182 NEW ZEALAND: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 183 NEW ZEALAND: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 184 NEW ZEALAND: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 185 NEW ZEALAND: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 186 NEW ZEALAND: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 187 REST OF ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 188 REST OF ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 189 REST OF ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 190 REST OF ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 191 REST OF ASIA PACIFIC: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 192 LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION
- TABLE 193 LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 194 LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 195 LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 196 LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 197 LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 198 BRAZIL: KEY MACROINDICATORS
- TABLE 199 BRAZIL: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 200 BRAZIL: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 201 BRAZIL: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 202 BRAZIL: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 203 BRAZIL: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 204 MEXICO: KEY MACROINDICATORS
- TABLE 205 MEXICO: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 206 MEXICO: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 207 MEXICO: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 208 MEXICO: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 209 MEXICO: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 210 REST OF LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 211 REST OF LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 212 REST OF LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 213 REST OF LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 214 REST OF LATIN AMERICA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 215 MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 216 MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 217 MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 218 MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 219 MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 220 MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 221 GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 222 GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 223 GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 224 GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 225 GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 226 GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 227 KINGDOM OF SAUDI ARABIA: KEY MACROINDICATORS
- TABLE 228 KINGDOM OF SAUDI ARABIA (KSA): VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 229 KINGDOM OF SAUDI ARABIA (KSA): VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 230 KINGDOM OF SAUDI ARABIA (KSA): VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 231 KINGDOM OF SAUDI ARABIA (KSA): VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 232 KINGDOM OF SAUDI ARABIA (KSA): VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 233 UNITED ARAB EMIRATES (UAE): KEY MACROINDICATORS
- TABLE 234 UNITED ARAB EMIRATES (UAE): VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 235 UNITED ARAB EMIRATES (UAE): VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 236 UNITED ARAB EMIRATES (UAE): VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 237 UNITED ARAB EMIRATES (UAE): VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 238 UNITED ARAB EMIRATES (UAE): VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 239 REST OF GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 240 REST OF GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 241 REST OF GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 242 REST OF GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 243 REST OF GCC COUNTRIES: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 244 REST OF MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2023-2030 (USD MILLION)
- TABLE 245 REST OF MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2023-2030 (USD MILLION)
- TABLE 246 REST OF MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
- TABLE 247 REST OF MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 248 REST OF MIDDLE EAST & AFRICA: VETERINARY BIOLOGICS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 249 OVERVIEW OF STRATEGIES DEPLOYED BY KEY PLAYERS IN VETERINARY BIOLOGICS MARKET
- TABLE 250 VETERINARY BIOLOGICS MARKET: DEGREE OF COMPETITION, 2024
- TABLE 251 VETERINARY BIOLOGICS MARKET: DEGREE OF COMPETITION, 2024
- TABLE 252 VETERINARY DIAGNOSTIC KITS MARKET: DEGREE OF COMPETITION, 2024
- TABLE 254 VETERINARY BIOLOGICS MARKET: REGION FOOTPRINT
- TABLE 255 VETERINARY BIOLOGICS MARKET: PRODUCT TYPE FOOTPRINT
- TABLE 256 VETERINARY BIOLOGICS MARKET: ANIMAL TYPE FOOTPRINT
- TABLE 257 VETERINARY BIOLOGICS MARKET: APPLICATION FOOTPRINT
- TABLE 258 VETERINARY BIOLOGICS MARKET: DETAILED LIST OF KEY STARTUP/SME PLAYERS
- TABLE 259 VETERINARY BIOLOGICS MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SME PLAYERS, BY ANIMAL TYPE AND REGION
- TABLE 260 VETERINARY BIOLOGICS MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-AUGUST 2025
- TABLE 261 VETERINARY BIOLOGICS MARKET: DEALS, JANUARY 2022-AUGUST 2025
- TABLE 262 VETERINARY BIOLOGICS MARKET: EXPANSIONS, JANUARY 2022-AUGUST 2025
- TABLE 263 VETERINARY BIOLOGICS MARKET: OTHER DEVELOPMENTS, JANUARY 2022-AUGUST 2025
- TABLE 264 ZOETIS SERVICES LLC: COMPANY OVERVIEW
- TABLE 265 ZOETIS SERVICES LLC: PRODUCTS OFFERED
- TABLE 266 ZOETIS SERVICES LLC: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-AUGUST 2025
- TABLE 267 ZOETIS SERVICES LLC: DEALS, JANUARY 2022-AUGUST 2025
- TABLE 268 ZOETIS SERVICES LLC: EXPANSIONS, JANUARY 2022-AUGUST 2025
- TABLE 269 ZOETIS SERVICES LLC: OTHER DEVELOPMENTS, JANUARY 2022-AUGUST 2025
- TABLE 270 IDEXX: COMPANY OVERVIEW
- TABLE 271 IDEXX: PRODUCTS OFFERED
- TABLE 272 IDEXX: PRODUCT LAUNCHES, JANUARY 2022-AUGUST 2025
- TABLE 273 ELANCO: COMPANY OVERVIEW
- TABLE 274 ELANCO: PRODUCTS OFFERED
- TABLE 275 ELANCO: PRODUCT LAUNCHES, JANUARY 2022-AUGUST 2025
- TABLE 276 ELANCO: DEALS, JANUARY 2022-AUGUST 2025
- TABLE 277 ELANCO: EXPANSIONS, JANUARY 2022-AUGUST 2025
- TABLE 278 MERCK & CO., INC.: COMPANY OVERVIEW
- TABLE 279 MERCK & CO., INC.: PRODUCTS OFFERED
- TABLE 280 MERCK & CO., INC.: PRODUCT LAUNCHES, JANUARY 2022-AUGUST 2025
- TABLE 281 MERCK & CO., INC.: EXPANSIONS, JANUARY 2022-AUGUST 2025
- TABLE 282 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
- TABLE 283 THERMO FISHER SCIENTIFIC INC.: PRODUCTS OFFERED
- TABLE 284 BOEHRINGER INGELHEIM INTERNATIONAL GMBH: COMPANY OVERVIEW
- TABLE 285 BOEHRINGER INGELHEIM INTERNATIONAL GMBH: PRODUCTS OFFERED
- TABLE 286 BOEHRINGER INGELHEIM INTERNATIONAL GMBH: DEALS, JANUARY 2022-AUGUST 2025
- TABLE 287 BOEHRINGER INGELHEIM INTERNATIONAL GMBH: EXPANSIONS, JANUARY 2022-AUGUST 2025
- TABLE 288 NEOGEN CORPORATION: COMPANY OVERVIEW
- TABLE 289 NEOGEN CORPORATION: PRODUCTS OFFERED
- TABLE 290 NEOGEN CORPORATION: DEALS, JANUARY 2022-AUGUST 2025
- TABLE 291 BIO-RAD LABORATORIES, INC.: COMPANY OVERVIEW
- TABLE 292 BIO-RAD LABORATORIES, INC.: PRODUCTS OFFERED
- TABLE 293 IMMUCELL CORPORATION: COMPANY OVERVIEW
- TABLE 294 IMMUCELL CORPORATION: PRODUCTS OFFERED
- TABLE 295 IMMUCELL CORPORATION: OTHER DEVELOPMENTS, JANUARY 2022-AUGUST 2025
- TABLE 296 INNOVATIVE DIAGNOSTICS: COMPANY OVERVIEW
- TABLE 297 INNOVATIVE DIAGNOSTICS: PRODUCTS OFFERED
- TABLE 298 BIONOTE USA INC.: COMPANY OVERVIEW
- TABLE 299 BIOCHECK: COMPANY OVERVIEW
- TABLE 300 PLASVACC USA: COMPANY OVERVIEW
- TABLE 301 NOVAVIVE INC.: COMPANY OVERVIEW
- TABLE 302 MG BIOLOGICS: COMPANY OVERVIEW
- TABLE 303 STALLERGENES GREER: COMPANY OVERVIEW
- TABLE 304 BIOSTONE ANIMAL HEALTH: COMPANY OVERVIEW
- TABLE 305 COLORADO SERUM COMPANY: COMPANY OVERVIEW
- TABLE 306 PADULA SERUMS PTY LTD.: COMPANY OVERVIEW
- TABLE 307 LAKE IMMUNOGENICS: COMPANY OVERVIEW
- TABLE 308 INBIOS INTERNATIONAL, INC.: COMPANY OVERVIEW
- TABLE 309 PRINCETON BIOMEDITECH CORPORATION: COMPANY OVERVIEW
- TABLE 310 TETRACORE, INC.: COMPANY OVERVIEW
- TABLE 311 VMRD, INC.: COMPANY OVERVIEW
- TABLE 312 ANIMAB: COMPANY OVERVIEW
List of Figures
- FIGURE 1 VETERINARY BIOLOGICS MARKET SEGMENTATION & REGIONAL SCOPE
- FIGURE 2 VETERINARY BIOLOGICS MARKET: YEARS CONSIDERED
- FIGURE 3 VETERINARY BIOLOGICS MARKET: RESEARCH DESIGN
- FIGURE 4 VETERINARY BIOLOGICS MARKET: KEY DATA FROM SECONDARY SOURCES
- FIGURE 5 VETERINARY BIOLOGICS MARKET: KEY PRIMARY SOURCES
- FIGURE 6 VETERINARY BIOLOGICS MARKET: INSIGHTS FROM KEY PRIMARIES
- FIGURE 7 BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY- AND DEMAND-SIDE PARTICIPANTS
- FIGURE 8 BREAKDOWN OF PRIMARY INTERVIEWS (SUPPLY SIDE): BY COMPANY TYPE, DESIGNATION, AND REGION
- FIGURE 9 BREAKDOWN OF PRIMARY INTERVIEWS (DEMAND SIDE): BY END USER, DESIGNATION, AND REGION
- FIGURE 10 VETERINARY BIOLOGICS MARKET SIZE ESTIMATION: SUPPLY-SIDE ANALYSIS (2024)
- FIGURE 11 VETERINARY BIOLOGICS MARKET SIZE ESTIMATION
- FIGURE 12 VETERINARY BIOLOGICS MARKET: TOP-DOWN APPROACH
- FIGURE 13 VETERINARY BIOLOGICS MARKET: BOTTOM-UP APPROACH
- FIGURE 14 VETERINARY BIOLOGICS MARKET: CAGR PROJECTIONS
- FIGURE 15 VETERINARY BIOLOGICS MARKET: DATA TRIANGULATION METHODOLOGY
- FIGURE 16 VETERINARY BIOLOGICS MARKET, BY PRODUCT TYPE, 2025 VS. 2030 (USD MILLION)
- FIGURE 17 VETERINARY BIOLOGICS MARKET, BY ANIMAL TYPE, 2025 VS. 2030 (USD MILLION)
- FIGURE 18 VETERINARY BIOLOGICS MARKET, BY ROUTE OF ADMINISTRATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 19 VETERINARY BIOLOGICS MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 20 VETERINARY BIOLOGICS MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- FIGURE 21 GEOGRAPHICAL SNAPSHOT OF VETERINARY BIOLOGICS MARKET
- FIGURE 22 INCREASING PREVALENCE OF CHRONIC DISEASES AMONG COMPANION ANIMALS TO DRIVE MARKET
- FIGURE 23 US AND COMPANION ANIMALS COMMANDED LARGEST NORTH AMERICAN VETERINARY BIOLOGICS MARKET SHARE IN 2024
- FIGURE 24 MONOCLONAL ANTIBODIES CAPTURED LARGEST SHARE DURING FORECAST PERIOD
- FIGURE 25 COMPANION ANIMALS TO ACCOUNT FOR LARGEST MARKET SHARE IN 2030
- FIGURE 26 INJECTABLE ROUTE OF ADMINISTRATION TO DOMINATE MARKET IN 2030
- FIGURE 27 DERMATOLOGY TO LEAD VETERINARY BIOLOGICS MARKET IN 2030
- FIGURE 28 US TO REGISTER HIGHEST GROWTH IN VETERINARY BIOLOGICS MARKET FROM 2025 TO 2030
- FIGURE 29 VETERINARY BIOLOGICS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 30 VETERINARY BIOLOGICS MARKET: TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 31 VETERINARY BIOLOGICS MARKET: VALUE CHAIN ANALYSIS
- FIGURE 32 VETERINARY BIOLOGICS MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 33 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS, BY END USER
- FIGURE 34 KEY BUYING CRITERIA FOR MAJOR END USERS
- FIGURE 35 PATENT ANALYSIS FOR VETERINARY MONOCLONAL ANTIBODIES IN VETERINARY BIOLOGICS MARKET, JANUARY 2014-AUGUST 2025
- FIGURE 36 TOP APPLICANT COUNTRIES AND NUMBER OF PATENT APPLICANTS FOR VETERINARY MONOCLONAL ANTIBODIES, BY JURISDICTION, JANUARY 2014-AUGUST 2025
- FIGURE 37 VETERINARY BIOLOGICS MARKET: ECOSYSTEM ANALYSIS
- FIGURE 38 KEY AI USE CASES IN VETERINARY BIOLOGICS MARKET
- FIGURE 39 FUNDING AND NUMBER OF DEALS IN VETERINARY BIOLOGICS MARKET, 2020-2024 (USD MILLION)
- FIGURE 40 NORTH AMERICA: VETERINARY BIOLOGICS MARKET SNAPSHOT
- FIGURE 41 REVENUE ANALYSIS OF KEY PLAYERS IN VETERINARY BIOLOGICS MARKET, 2022-2024
- FIGURE 42 MARKET SHARE ANALYSIS OF KEY PLAYERS IN VETERINARY BIOLOGICS MARKET (2024)
- FIGURE 43 US MARKET SHARE ANALYSIS OF KEY PLAYERS IN VETERINARY BIOLOGICS MARKET (2024)
- FIGURE 44 MARKET SHARE ANALYSIS OF KEY PLAYERS IN VETERINARY DIAGNOSTIC KITS MARKET (2024)
- FIGURE 45 US: VETERINARY DIAGNOSTIC KITS MARKET SHARE ANALYSIS, 2024
- FIGURE 46 RANKING OF KEY PLAYERS IN VETERINARY BIOLOGICS MARKET (2024)
- FIGURE 47 RANKING OF KEY PLAYERS IN VETERINARY DIAGNOSTIC KITS MARKET (2024)
- FIGURE 48 VETERINARY BIOLOGICS MARKET: COMPANY EVALUATION MATRIX, 2024
- FIGURE 49 VETERINARY BIOLOGICS MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 50 VETERINARY BIOLOGICS MARKET: BRAND/PRODUCT COMPARATIVE ANALYSIS
- FIGURE 51 EV/EBITDA OF KEY VENDORS
- FIGURE 52 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 53 ZOETIS SERVICES LLC: COMPANY SNAPSHOT
- FIGURE 54 IDEXX: COMPANY SNAPSHOT
- FIGURE 55 ELANCO: COMPANY SNAPSHOT
- FIGURE 56 MERCK & CO., INC.: COMPANY SNAPSHOT
- FIGURE 57 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT
- FIGURE 58 BOEHRINGER INGELHEIM INTERNATIONAL GMBH: COMPANY SNAPSHOT
- FIGURE 59 NEOGEN CORPORATION: COMPANY SNAPSHOT
- FIGURE 60 BIO-RAD LABORATORIES, INC.: COMPANY SNAPSHOT
- FIGURE 61 IMMUCELL CORPORATION: COMPANY SNAPSHOT







