 |
市場調查報告書
商品編碼
1777131
全球藥用輔料市場(按產品、功能、劑型、功能用途和地區分類)- 預測至 2030 年Pharmaceutical Excipients Market by Product ((Organic, Inorganic)), Functionality, Formulation, Functionality Application - Global Forecast to 2030 |
||||||
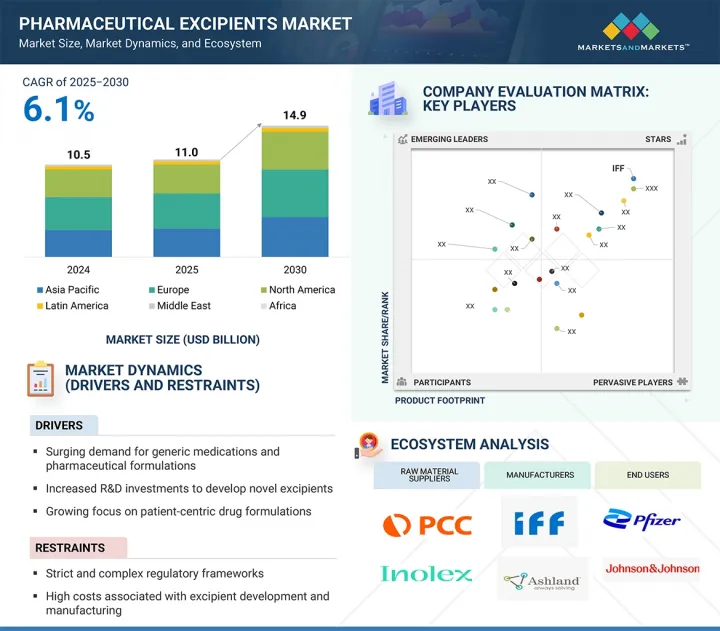
全球藥用輔料市場規模預計將從 2025 年的 110.3 億美元成長到 2030 年的 148.6 億美元,預測期內的複合年成長率為 6.1%。
| 研究範圍 | |
|---|---|
| 調查年份 | 2023-2030 |
| 基準年 | 2024 |
| 預測期 | 2025-2030 |
| 對價單位 | 金額(十億美元) |
| 按細分市場 | 依產品、功能、劑型、功能用途、地區 |
| 目標區域 | 北美、歐洲、亞太地區、拉丁美洲、中東和非洲 |
預計未來幾年藥用輔料市場將持續擴張,主要驅動力在於對藥品和學名藥日益成長的需求,以及對新型輔料研發的投入。此外,與製藥公司的合作與夥伴關係,以及對以患者為中心的配方日益重視,預計將帶來豐厚的市場成長機會。然而,嚴格的監管和高昂的開發成本預計將限制市場的發展。
藥用輔料市場可依功能分類,包括粘合劑、懸浮劑和黏度劑、填充劑和稀釋劑、調味劑和甜味劑、著色劑、被覆劑、潤滑劑、防腐劑、崩壞、乳化劑和其他特殊劑。其中,填充劑和稀釋劑在固態口服劑型(尤其是錠劑和膠囊)的生產中起著至關重要的作用。隨著口服藥物需求的不斷成長,它們的重要性持續成長,口服藥物由於給藥方便、患者依從性高仍然是最受歡迎的途徑。填充劑和稀釋劑可以增加含有少量活性藥物成分的配方的體積,有助於提高劑量的準確性和含量均勻性。從製造角度來看,它們對配方穩定性、易於加工和大規模生產的擴充性做出了顯著貢獻。它們還簡化了複合、壓片和封裝等工藝,同時提高了錠劑的機械強度。對於患者而言,這些輔料可以改善口感、口味和操作性,而這些因素對兒科和老年患者至關重要。隨著藥物配方日益複雜,對支持控制釋放和提高生物有效性度的高性能、多功能填充劑和稀釋劑的需求也在不斷成長,這為此類輔料創造了巨大的成長機會。
藥用輔料市場按劑型可分為口服劑型、外用劑型、腸外劑型及其他劑型。其中,口服劑型在2024年佔據了最大的市場佔有率,反映出其作為最廣泛使用的傳統給藥方式的主導地位。口服給藥因其便捷性、成本效益和患者依從性高而受到患者和醫療保健提供者的青睞。由於對錠劑、膠囊和其他固態口服劑型的需求不斷成長,該細分市場持續擴張。此外,生物製藥和學名藥行業的快速發展在推動對高品質輔料的需求方面發揮關鍵作用,這些輔料可提高口服產品的藥物穩定性、溶解度和生物有效性。需要長期服藥的慢性病的增加以及自我服藥的趨勢也促進了該細分市場的成長。因此,製藥公司正在投資創新口服劑型,從而推動了對先進輔料的需求以滿足這些需求。
預計亞太地區藥用輔料市場將強勁成長,在預測期內的複合年成長率位居所有地區之首。多種相互關聯的因素正在推動這一擴張。其中一個關鍵優勢是該地區經濟高效的製造能力,使其成為製藥製造和外包的理想之地。印度、中國和韓國等國家擁有豐富的原料、熟練的勞動力以及支持醫藥研發和出口導向製造業的優惠政府政策。此外,與新興市場相比,亞太地區的法律規範相對寬鬆,從而加快了產品開發和上市速度。人均收入的快速成長、健康意識的增強以及醫療保健支出的不斷增加,進一步推動了對學名藥的需求,進而也推動了藥用輔料的需求。此外,與生活方式相關的慢性病的快速成長和老年人口的不斷成長,也推動了對創新藥物製劑的需求,包括口服固態和外用製劑。這些趨勢為區域和全球輔料製造商提供了重大機遇,使其能夠擴大業務規模、投資基礎設施並建立策略夥伴關係,以滿足日益成長的區域需求。
本報告研究了全球藥用輔料市場,並提供了按產品、功能、劑型、功能用途和地區分類的趨勢資訊,以及參與市場的公司概況。
目錄
第1章 引言
第2章調查方法
第3章執行摘要
第4章重要考察
第5章市場概述
- 介紹
- 市場動態
- 影響客戶業務的趨勢/中斷
- 定價分析
- 價值鏈分析
- 供應鏈分析
- 生態系分析
- 投資金籌措場景
- 技術分析
- 專利分析
- 貿易分析
- 2025-2026 年重要會議與活動
- 監管狀況
- 波特五力分析
- 主要相關人員和採購標準
- 人工智慧/產生人工智慧對藥用輔料市場的影響
- 2025年美國關稅對藥用輔料市場的影響
第6章藥用輔料市場(依產品)
- 介紹
- 有機化學品
- 無機化學品
- 其他化學品
第7章藥用輔料市場(依功能)
- 介紹
- 填充劑和稀釋劑
- 懸浮劑和黏度劑
- 被覆劑
- 粘合劑
- 調味劑和甜味劑
- 崩壞
- 色素
- 潤滑劑
- 防腐劑
- 乳化劑
- 其他
第8章 藥用輔料市場(以劑型)
- 介紹
- 口服
- 局部藥物
- 腸外
- 其他
第9章藥用輔料市場(依功能用途)
- 介紹
- 穩定劑
- 遮罩味
- 改良釋放
- 提高溶解度和生物有效性
- 其他
第10章 藥用輔料市場(按地區)
- 介紹
- 歐洲
- 歐洲宏觀經濟分析
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 其他
- 北美洲
- 北美宏觀經濟分析
- 美國
- 加拿大
- 亞太地區
- 亞太宏觀經濟分析
- 中國
- 日本
- 印度
- 韓國
- 其他
- 拉丁美洲
- 人口老化和疾病發病率上升將推動市場成長
- 拉丁美洲宏觀經濟分析
- 巴西
- 墨西哥
- 其他
- 中東
- 為了振興市場,人們開始關注不飽和的市場。
- 中東宏觀經濟分析
- 海灣合作理事會國家
- 其他
- 非洲
- 大型製藥公司影響力增強,推動成長
- 非洲宏觀經濟分析
第11章競爭格局
- 介紹
- 主要參與企業的策略/優勢
- 2020-2024年收益分析
- 2024年市場佔有率分析
- 估值和財務指標
- 品牌/產品比較
- 公司估值矩陣:2024 年關鍵參與企業
- 公司估值矩陣:Start-Ups/中小企業,2024 年
- 競爭場景
第12章:公司簡介
- 主要參與企業
- INTERNATIONAL FLAVORS & FRAGRANCES INC.
- ADM
- ROQUETTE FRERES
- BASF SE
- EVONIK
- ASHLAND
- KERRY GROUP PLC
- MERCK
- ASSOCIATED BRITISH FOODS PLC
- WACKER CHEMIE AG
- AIR LIQUIDE
- DOW
- BERKSHIRE HATHAWAY(LUBRIZOL CORPORATION)
- COLORCON, INC.
- DFE PHARMA
- ACTYLIS
- CRODA INTERNATIONAL PLC
- CHEMIE TRADE
- GATTEFOSSE
- J. RETTENMAIER & SOHNE GMBH+CO KG
- SHIN-ETSU CHEMICAL CO., LTD.
- 其他公司
- INNOPHOS
- MEGGLE GMBH & CO. KG
- FUJI CHEMICAL INDUSTRIES CO., LTD.
- COREL PHARMA CHEM
- BIOGRUND
- NITIKA PHARMACEUTICAL SPECIALTIES PVT. LTD.
- RT VANDERBILT HOLDING COMPANY, INC.
- BENEO
- SIAGACHI INDUSTRIES
- JH NANHANG LIFE SCIENCES CO., LTD.
第13章 附錄
The global pharmaceutical excipients market is projected to reach USD 14.86 billion by 2030 from USD 11.03 billion in 2025, at a CAGR of 6.1% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | By Product, Functionality, Formulation, Functionality, and Application |
| Regions covered | North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa |
The expansion of the Pharmaceutical Excipients market has been predominantly driven by the Surging demand for pharmaceutical products and generic medicines, and R&D investments for novel excipient development are predicted to uplift the market in the coming years. Additionally, partnerships and collaboration with pharmaceutical companies and increased emphasis on patient-centric formulations will provide lucrative market growth opportunities. However, regulatory stringency and steep development costs are predicted to restrict the market.

The fillers & diluents functionality held the highest share in the pharmaceutical excipients market.
The pharmaceutical excipients market, segmented by functionality, includes binders, suspending and viscosity agents, fillers and diluents, flavoring agents and sweeteners, colorants, coating agents, lubricants and glidants, preservatives, disintegrants, emulsifying agents, and other specialized roles. Among these, fillers and diluents play a critical role, especially in the production of solid oral dosage forms like tablets and capsules. Their importance continues to grow with the rising demand for oral medications, which remain the most preferred route due to ease of administration and patient compliance. Fillers and diluents provide bulk to formulations containing small amounts of active pharmaceutical ingredients and help ensure accurate dosage and improved content uniformity. From a manufacturing perspective, they contribute significantly to formulation stability, ease of processing, and scalability in mass production. They simplify processes such as blending, tableting, and encapsulation while enhancing the mechanical strength of tablets. For patients, these excipients improve mouthfeel, taste, and handling, which are crucial factors for pediatric and geriatric populations. As drug formulations become more complex, the demand for high-performance, multifunctional fillers and diluents that support controlled release and bioavailability enhancement is also rising, presenting significant growth opportunities in this excipient category.
The oral formulations segment reported the highest share of the formulations segment in 2024.
The pharmaceutical excipients market is categorized based on formulation types into oral formulations, topical formulations, parenteral formulations, and other formulations. Among these, the oral formulations segment held the largest market share in 2024, reflecting its dominance as the most widely used and traditional drug delivery method. The convenience, cost-effectiveness, and high patient compliance associated with oral administration make it a preferred choice for patients and healthcare providers. This segment continues to expand due to the rising demand for tablets, capsules, and other solid oral dosage forms. Furthermore, the rapid growth of the biopharmaceutical and generics industries is playing a pivotal role in driving demand for high-quality excipients that enhance drug stability, solubility, and bioavailability in oral products. The increasing prevalence of chronic conditions requiring long-term medication and the trend toward self-administration of drugs also contribute to the growth of this segment. As a result, pharmaceutical companies are investing in innovative oral drug formulations, boosting the need for advanced excipients tailored to these needs.
Asia Pacific is expected to grow at the highest CAGR in the global pharmaceutical excipients market from 2025 to 2030.
The pharmaceutical excipients market in the Asia Pacific region is expected to grow robustly and register the highest CAGR among all regions during the forecast period. Several interrelated factors are driving this expansion. One of the key advantages is the region's cost-effective manufacturing capabilities, which make it an attractive hub for pharmaceutical production and outsourcing. Countries like India, China, and South Korea offer abundant raw material availability, skilled labor, and favorable government policies that support pharmaceutical R&D and export-oriented manufacturing. Additionally, the Asia Pacific region has relatively lenient regulatory frameworks compared to Western markets, which accelerates product development and time-to-market. The rapid rise in per capita income, growing awareness of health and wellness, and increased healthcare spending are further fueling demand for generic drugs and, by extension, pharmaceutical excipients. Moreover, a surge in lifestyle-related chronic diseases and expanding geriatric populations have led to greater demand for innovative drug formulations, including oral solids and topical applications. These trends create significant opportunities for local and global excipient manufacturers to expand operations, invest in infrastructure, and form strategic partnerships to meet the growing regional demand.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side-70% and Demand Side-30%
- By Designation: Managers-45%, CXOs and Directors-30%, and Executives-25%
- By Region: North America-40%, Europe-25%, the Asia Pacific-25%, Latin America-5%, and the Middle East & Africa-5%
List of Key Companies Profiled in the Report
Key players in the pharmaceutical excipients market include International Flavors & Fragrances Inc. (US), Ashland Inc. (US), Evonik Industries AG (Germany), BASF SE (Germany), Kerry Group plc (Ireland), Roquette Freres (France), MERCK KGaA (Germany), Associated British Foods plc (UK), ADM (US), Wacker Chemie AG (Germany), Air Liquide (France), DOW (US), Berkshire Hathaway Inc. (US), Colorcon (US), DFE PHARMA (Germany), Actylis (US), Croda International Plc(UK), Chemie Trade(India), Gattefosse (France), JRS Pharma(US), and Shin-Etsu Chemical Co., Ltd. (Japan).
Research Coverage
This research report categorizes the pharmaceutical excipients market by Product [(Organic (Oleochemicals, Carbohydrates, Petrochemicals, Proteins, and Other Organic Chemicals), Inorganic (Calcium Phosphate, Metal Oxides, Halites, Calcium Carbonate, Calcium Sulfate, Other Inorganic Chemicals), Other chemicals], by Functionality (Fillers & Diluents, Suspending & Viscosity Agents, Coating Agents, Binders, Flavoring Agents & Sweeteners, Disintegrants, Colorants, Lubricants & Glidants, Preservatives, Emulsifying Agents, and Other Functionalities), by formulation (Oral (Tablets, Capsules, Liquid Formulations and other oral formulations), Topical Formulations, Parenteral Formulations, and Other Formulations), by Functionality Application (Stabilizers, Taste Making, Modified Release, Solubility & Bioavailability Enhancement and Other Functionality Applications), and by Region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa).
The scope of the report covers detailed information regarding the major factors, such as drivers, restraints, challenges, and opportunities, influencing the growth of the pharmaceutical excipients market. A detailed analysis of the key industry players has been done to provide insights into their business overview, products, solutions, key strategies, collaborations, partnerships, and agreements. New approvals/launches, collaborations, acquisitions, and recent developments associated with the pharmaceutical excipients market.
Reasons to Buy this Report
The report will help market leaders and new entrants by providing them with the closest approximations of the revenue numbers for the overall pharmaceutical excipients market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their businesses and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (Surging demand for generic medications and pharmaceutical formulations, Increased R&D investments to develop novel excipients, A growing focus on patient-centric drug formulations, Strategic collaborations and partnerships with pharmaceutical firms), restraints (Strict and complex regulatory frameworks, High costs associated with excipient development and manufacturing), opportunities (Increasing interest in functional and multifunctional excipients, Technological advancements, particularly in nanotechnology, Expansion into emerging economies across Asia Pacific and Latin America), and Challenges (Ongoing concerns about excipient safety and quality standards, Limited availability and fluctuating costs of key raw materials).
- Product Development/Innovation: Detailed insights on upcoming products, research and development activities, and new product approvals/launches in the Pharmaceutical Excipients market.
- Market Development: Comprehensive information about lucrative markets; the report analyses the market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the pharmaceutical excipients market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players. A detailed analysis of the key industry players has been done to provide insights into their key strategies, product launches/ approvals, acquisitions, partnerships, agreements, collaborations, other recent developments, investment and funding activities, brand/product comparative analysis, and vendor valuation and financial metrics of the pharmaceutical excipients market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION
- 1.3.2 REGIONAL SCOPE
- 1.3.3 INCLUSIONS AND EXCLUSIONS
- 1.3.4 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Objectives of secondary research
- 2.1.1.2 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Breakdown of primary interviews
- 2.1.2.2 Key objectives of primary research
- 2.1.1 SECONDARY DATA
- 2.2 MARKET ESTIMATION
- 2.2.1 GLOBAL MARKET ESTIMATION
- 2.2.1.1 Company revenue analysis (Bottom-up approach)
- 2.2.1.2 Revenue share analysis
- 2.2.1.3 MnM repository analysis
- 2.2.1.4 Primary interviews
- 2.2.2 INSIGHTS FROM PRIMARY EXPERTS
- 2.2.3 SEGMENTAL MARKET SIZE ESTIMATION (TOP-DOWN APPROACH)
- 2.2.1 GLOBAL MARKET ESTIMATION
- 2.3 GROWTH RATE PROJECTIONS
- 2.4 DATA TRIANGULATION
- 2.5 RESEARCH ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 PHARMACEUTICAL EXCIPIENTS MARKET OVERVIEW
- 4.2 NORTH AMERICA PHARMACEUTICAL EXCIPIENTS MARKET
- 4.3 PHARMACEUTICAL EXCIPIENTS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.4 PHARMACEUTICAL EXCIPIENTS MARKET: EMERGING VS. DEVELOPED MARKETS, 2025 VS. 2030 (USD MILLION)
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Surging demand for generic medications and pharmaceutical formulations.
- 5.2.1.2 Increased R&D investments to develop novel excipients
- 5.2.1.3 Growing focus on patient-centric drug formulations
- 5.2.1.4 Strategic collaborations and partnerships among pharmaceutical firms
- 5.2.2 RESTRAINTS
- 5.2.2.1 Strict and complex regulatory frameworks
- 5.2.2.2 High cost of excipient development and manufacturing
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Increasing interest in functional and multifunctional excipients
- 5.2.3.2 Technological advancements in nanotechnology
- 5.2.3.3 Market opportunities in emerging economies
- 5.2.4 CHALLENGES
- 5.2.4.1 Ongoing concerns about excipient safety and quality standards
- 5.2.4.2 Limited availability and fluctuating costs of key raw materials
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.4 PRICING ANALYSIS
- 5.4.1 INDICATIVE PRICING ANALYSIS, BY KEY PLAYER
- 5.4.2 INDICATIVE PRICING ANALYSIS, BY REGION
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- 5.8 INVESTMENT AND FUNDING SCENARIO
- 5.9 TECHNOLOGY ANALYSIS
- 5.9.1 KEY TECHNOLOGIES
- 5.9.1.1 Co-processed excipient technology
- 5.9.1.2 Nanotechnology-based excipients
- 5.9.2 COMPLEMENTARY TECHNOLOGIES
- 5.9.2.1 3D printing/additive manufacturing
- 5.9.2.2 Process Analytical Technology (PAT)
- 5.9.3 ADJACENT TECHNOLOGIES
- 5.9.3.1 Artificial intelligence in formulation design
- 5.9.3.2 Green chemistry/sustainability tech
- 5.9.1 KEY TECHNOLOGIES
- 5.10 PATENT ANALYSIS
- 5.11 TRADE ANALYSIS
- 5.11.1 TRADE DATA FOR HS CODE 290545
- 5.11.1.1 Import data for HS code 290545
- 5.11.1.2 Export data for HS code 290545
- 5.11.2 TRADE DATA FOR HS CODE 350510
- 5.11.2.1 Import data for HS code 350510
- 5.11.2.2 Export data for HS code 350510
- 5.11.3 TRADE DATA FOR HS CODE 290532
- 5.11.3.1 Import data for HS code 290532
- 5.11.3.2 Export data for HS code 290532
- 5.11.4 TRADE DATA FOR HS CODE 283650
- 5.11.4.1 Import data for HS code 283650
- 5.11.4.2 Export data for HS code 283650
- 5.11.5 TRADE DATA FOR HS CODE 250100
- 5.11.5.1 Import data for HS code 250100
- 5.11.5.2 Export data for HS code 250100
- 5.11.6 TRADE DATA FOR HS CODE 290543
- 5.11.6.1 Import data for HS code 290543
- 5.11.6.2 Export data for HS code 290543
- 5.11.7 TRADE DATA FOR HS CODE 3912
- 5.11.7.1 Import data for HS code 3912
- 5.11.7.2 Export data for HS code 3912
- 5.11.1 TRADE DATA FOR HS CODE 290545
- 5.12 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.13 REGULATORY LANDSCAPE
- 5.13.1 REGULATORY FRAMEWORK
- 5.13.1.1 US
- 5.13.1.2 Europe
- 5.13.1.3 China
- 5.13.1.4 India
- 5.13.1.5 Brazil
- 5.13.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.13.1 REGULATORY FRAMEWORK
- 5.14 PORTER'S FIVE FORCES ANALYSIS
- 5.14.1 BARGAINING POWER OF SUPPLIERS
- 5.14.2 BARGAINING POWER OF BUYERS
- 5.14.3 THREAT OF NEW ENTRANTS
- 5.14.4 THREAT OF SUBSTITUTES
- 5.14.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.15 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.15.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.15.2 KEY BUYING CRITERIA
- 5.16 IMPACT OF AI/GEN AI ON PHARMACEUTICAL EXCIPIENTS MARKET
- 5.16.1 INTRODUCTION
- 5.16.2 MARKET POTENTIAL OF AI ON PHARMACEUTICAL EXCIPIENTS
- 5.16.3 AI USE CASES
- 5.16.4 KEY COMPANIES IMPLEMENTING AI
- 5.16.5 FUTURE OF GENERATIVE AI IN PHARMACEUTICAL EXCIPIENT ECOSYSTEM
- 5.17 IMPACT OF 2025 US TARIFF ON PHARMACEUTICAL EXCIPIENTS MARKET
- 5.17.1 INTRODUCTION
- 5.17.2 KEY TARIFF RATES
- 5.17.3 PRICE IMPACT ANALYSIS
- 5.17.4 IMPACT ON COUNTRY/REGION
- 5.17.4.1 North America
- 5.17.4.1.1 US
- 5.17.4.2 Europe
- 5.17.4.3 Asia Pacific
- 5.17.4.1 North America
- 5.17.5 IMPACT ON END-USE INDUSTRIES
6 PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- 6.2 ORGANIC CHEMICALS
- 6.2.1 OLEOCHEMICALS
- 6.2.1.1 Fatty alcohols
- 6.2.1.1.1 Need for improved drug absorption to augment growth
- 6.2.1.2 Mineral stearates
- 6.2.1.2.1 Wide use of magnesium stearates as excipients in nutraceutical and pharmaceutical formulations to drive market
- 6.2.1.3 Glycerin
- 6.2.1.3.1 Non-toxic, colorless, and odorless properties to encourage growth
- 6.2.1.4 Other oleochemicals
- 6.2.1.1 Fatty alcohols
- 6.2.2 CARBOHYDRATES
- 6.2.2.1 Sugars
- 6.2.2.1.1 Actual sugars
- 6.2.2.1.1.1 Growing use of actual sugars in pediatric formulations to propel market
- 6.2.2.1.1.2 Lactose
- 6.2.2.1.1.3 Sucrose
- 6.2.2.1.1.4 Dextrose (D-Glucose)
- 6.2.2.1.2 Sugar alcohols
- 6.2.2.1.2.1 Surging trend in consumer demand for healthier alternatives to conventional sweeteners to fuel market
- 6.2.2.1.2.2 Sorbitol
- 6.2.2.1.2.3 Mannitol
- 6.2.2.1.2.4 Other sugar alcohols
- 6.2.2.1.3 Artificial sweeteners
- 6.2.2.1.3.1 Increasing use of sugar substitutes to favor growth
- 6.2.2.1.1 Actual sugars
- 6.2.2.2 Cellulose
- 6.2.2.2.1 Microcrystalline cellulose
- 6.2.2.2.1.1 Versatility, safety, and cost-effectiveness to facilitate growth
- 6.2.2.2.2 Cellulose ethers
- 6.2.2.2.2.1 Higher effectiveness than other water-soluble polymers to fuel market
- 6.2.2.2.3 CMC & croscarmellose sodium
- 6.2.2.2.3.1 Growing trend of sourcing CMC from more viable alternatives to boost market
- 6.2.2.2.4 Cellulose esters
- 6.2.2.2.4.1 Increasing focus on targeted and sustained-release drug delivery systems to sustain growth
- 6.2.2.2.1 Microcrystalline cellulose
- 6.2.2.3 Starch
- 6.2.2.3.1 Modified starch
- 6.2.2.3.1.1 Cold-water swelling and gel barrier formation capabilities to fuel market
- 6.2.2.3.2 Dried starch
- 6.2.2.3.2.1 Strong binding and coating properties to augment growth
- 6.2.2.3.3 Converted starch
- 6.2.2.3.3.1 Enhanced solubility and stability for drugs to foster growth
- 6.2.2.3.1 Modified starch
- 6.2.2.1 Sugars
- 6.2.3 PETROCHEMICALS
- 6.2.3.1 Glycols
- 6.2.3.1.1 Polyethylene glycol
- 6.2.3.1.1.1 Solubility, lubricity, and compatibility with APIs to drive market
- 6.2.3.1.2 Propylene glycol
- 6.2.3.1.2.1 Rising use of propylene glycol in oral and injectable formulations to foster growth
- 6.2.3.1.1 Polyethylene glycol
- 6.2.3.2 Povidones
- 6.2.3.2.1 Increasing demand for fast-dissolving tablets to spur growth
- 6.2.3.3 Mineral hydrocarbons
- 6.2.3.3.1 Petrolatum
- 6.2.3.3.1.1 Strong presence in topical pharmaceutical formulations to expedite growth
- 6.2.3.3.2 Mineral waxes
- 6.2.3.3.2.1 Utilization of mineral waxes in solid and semi-solid dosage to foster growth
- 6.2.3.3.3 Mineral oils
- 6.2.3.3.3.1 Extensive use of mineral oils as solvents and emollients to propel market
- 6.2.3.3.1 Petrolatum
- 6.2.3.4 Acrylic polymers
- 6.2.3.4.1 Wide use of acrylic polymers in controlled-release drug formulations to drive market
- 6.2.3.5 Other petrochemicals
- 6.2.3.1 Glycols
- 6.2.4 PROTEINS
- 6.2.4.1 Growing applications of proteins as carriers for microparticles and nanoparticles to propel market
- 6.2.5 OTHER ORGANIC CHEMICALS
- 6.2.1 OLEOCHEMICALS
- 6.3 INORGANIC CHEMICALS
- 6.3.1 CALCIUM PHOSPHATE
- 6.3.1.1 Chemical purity and low incompatibility with drugs to aid growth
- 6.3.2 METAL OXIDES
- 6.3.2.1 Availability of silicon oxides in the hydrophobic, hydrophilic, and granulated forms to aid market growth
- 6.3.3 HALITES
- 6.3.3.1 Increased utilization of halites in vaccines and controlled-release formulations to foster growth
- 6.3.4 CALCIUM CARBONATE
- 6.3.4.1 Short disintegration time and excellent mechanical strength to expedite growth
- 6.3.5 CALCIUM SULFATE
- 6.3.5.1 High quality and cost effectiveness to aid growth
- 6.3.6 OTHER INORGANIC CHEMICALS
- 6.3.1 CALCIUM PHOSPHATE
- 6.4 OTHER CHEMICALS
7 PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY
- 7.1 INTRODUCTION
- 7.2 FILLERS & DILUENTS
- 7.2.1 IMPROVED COHESION AND DIRECT COMPRESSION TO AMPLIFY GROWTH
- 7.3 SUSPENDING & VISCOSITY AGENTS
- 7.3.1 DRUG STABILITY AND TASTE MASKING ADVANTAGES TO SUSTAIN GROWTH
- 7.4 COATING AGENTS
- 7.4.1 INCREASING USE OF SUSTAINED-RELEASE FORMULATIONS IN PHARMA INDUSTRY TO DRIVE MARKET
- 7.5 BINDERS
- 7.5.1 HIGHER FLUIDITY AND COMPRESSIBILITY TO STIMULATE GROWTH
- 7.6 FLAVORING AGENTS & SWEETENERS
- 7.6.1 NEED TO IMPROVE DRUG PALATABILITY TO ADVANCE GROWTH
- 7.7 DISINTEGRANTS
- 7.7.1 NEED FOR RAPID BREAK-UP OF SOLID DOSAGE FORMS TO FOSTER GROWTH
- 7.8 COLORANTS
- 7.8.1 INCREASING USE OF COLORING IN HARD AND SOFT GELATIN CAPSULES, TABLETS, ORAL LIQUIDS, AND TOPICAL CREAMS TO BOOST MARKET
- 7.9 LUBRICANTS & GLIDANTS
- 7.9.1 NON-TOXIC PROPERTIES TO FAVOR GROWTH
- 7.10 PRESERVATIVES
- 7.10.1 GROWING USE OF PRESERVATIVES IN PHARMACEUTICAL AND NUTRACEUTICAL INDUSTRY TO DRIVE MARKET
- 7.11 EMULSIFYING AGENTS
- 7.11.1 RISING USE OF SOLUBILITY ENHANCEMENT EXCIPIENTS IN LIQUID DRUG FORMULATIONS TO PROMOTE GROWTH
- 7.12 OTHER FUNCTIONALITIES
8 PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION
- 8.1 INTRODUCTION
- 8.2 ORAL FORMULATIONS
- 8.2.1 TABLETS
- 8.2.1.1 High palatability and portability to support growth
- 8.2.2 CAPSULES
- 8.2.2.1 Hard-gelatin capsules
- 8.2.2.1.1 Ease of manufacturing and versatility to boost market
- 8.2.2.2 Soft-gelatin capsules
- 8.2.2.2.1 Quick-release properties to contribute to growth
- 8.2.2.1 Hard-gelatin capsules
- 8.2.3 LIQUID FORMULATIONS
- 8.2.3.1 Rapid absorption from stomach and intestines to foster growth
- 8.2.4 OTHER ORAL FORMULATIONS
- 8.2.1 TABLETS
- 8.3 TOPICAL FORMULATIONS
- 8.3.1 GROWING DEMAND FOR TRANSDERMAL PATCHES AND SELF-ADHERING TRANSDERMAL DRUG DELIVERY SYSTEMS TO FUEL MARKET
- 8.4 PARENTERAL FORMULATIONS
- 8.4.1 EMERGENCE OF BIOLOGICAL MOLECULES TO EXPEDITE GROWTH
- 8.5 OTHER FORMULATIONS
9 PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION
- 9.1 INTRODUCTION
- 9.2 STABILIZERS
- 9.2.1 IMPROVED DRUG SHELF LIFE AND LESSER MANUFACTURING DEFECTS TO PROMOTE GROWTH
- 9.3 TASTE MASKING
- 9.3.1 GROWING PREFERENCE FOR BETTER-TASTING SOLID DOSAGE FORMS TO PROPEL MARKET
- 9.4 MODIFIED-RELEASE
- 9.4.1 INCREASING USE OF MODIFIED-RELEASE FORMULATIONS IN PHARMA INDUSTRY TO FOSTER GROWTH
- 9.5 SOLUBILITY & BIOAVAILABILITY ENHANCEMENT
- 9.5.1 INCREASING PREFERENCE FOR SOLUBLE COMPOUNDS TO ACCELERATE GROWTH
- 9.6 OTHER APPLICATIONS
10 PHARMACEUTICAL EXCIPIENTS MARKET, BY REGION
- 10.1 INTRODUCTION
- 10.2 EUROPE
- 10.2.1 MACROECONOMIC ANALYSIS FOR EUROPE
- 10.2.2 GERMANY
- 10.2.2.1 Booming pharmaceutical manufacturing sector to aid growth
- 10.2.3 UK
- 10.2.3.1 Growing adoption of generics and development of novel excipients to fuel market
- 10.2.4 FRANCE
- 10.2.4.1 Large geriatric population to support growth
- 10.2.5 ITALY
- 10.2.5.1 Budgetary constraints and restricted healthcare spending to impede growth
- 10.2.6 SPAIN
- 10.2.6.1 High consumption of biologics to foster growth
- 10.2.7 REST OF EUROPE
- 10.3 NORTH AMERICA
- 10.3.1 MACROECONOMIC ANALYSIS FOR NORTH AMERICA
- 10.3.2 US
- 10.3.2.1 Well-developed healthcare infrastructure and research bases to aid growth
- 10.3.3 CANADA
- 10.3.3.1 Rising prevalence of cancer to contribute to growth
- 10.4 ASIA PACIFIC
- 10.4.1 MACROECONOMIC ANALYSIS FOR ASIA PACIFIC
- 10.4.2 CHINA
- 10.4.2.1 Emphasis on generics and off-patent drugs to drive market
- 10.4.3 JAPAN
- 10.4.3.1 Rapid growth of elderly population to augment growth
- 10.4.4 INDIA
- 10.4.4.1 High domestic demand for pharmaceuticals and export volume to boost market
- 10.4.5 SOUTH KOREA
- 10.4.5.1 Presence of leading contract research and development organizations to propel market
- 10.4.6 REST OF ASIA PACIFIC
- 10.5 LATIN AMERICA
- 10.5.1 GROWING GERIATRIC POPULATION AND RISING DISEASE PREVALENCE ARE DRIVING MARKET GROWTH
- 10.5.2 MACROECONOMIC ANALYSIS FOR LATIN AMERICA
- 10.5.3 BRAZIL
- 10.5.3.1 Emerging opportunities for excipient manufacturers to drive market
- 10.5.4 MEXICO
- 10.5.4.1 Growing demand for affordable therapies to boost market
- 10.5.5 REST OF LATIN AMERICA
- 10.6 MIDDLE EAST
- 10.6.1 GROWING FOCUS ON UNSATURATED MARKETS TO DRIVE MARKET
- 10.6.2 MACROECONOMIC ANALYSIS FOR MIDDLE EAST
- 10.6.3 GCC COUNTRIES
- 10.6.3.1 Expanding healthcare infrastructure expansion and localization of drug manufacturing to bolster growth
- 10.6.4 REST OF MIDDLE EAST
- 10.7 AFRICA
- 10.7.1 INCREASING PRESENCE OF MAJOR PHARMACEUTICAL COMPANIES TO AUGMENT GROWTH
- 10.7.2 MACROECONOMIC ANALYSIS FOR AFRICA
11 COMPETITIVE LANDSCAPE
- 11.1 INTRODUCTION
- 11.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN PHARMACEUTICAL EXCIPIENTS MARKET, 2022-2024
- 11.3 REVENUE ANALYSIS, 2020-2024
- 11.4 MARKET SHARE ANALYSIS, 2024
- 11.5 COMPANY VALUATION AND FINANCIAL METRICS
- 11.6 BRAND/PRODUCT COMPARISON
- 11.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 11.7.1 STARS
- 11.7.2 EMERGING LEADERS
- 11.7.3 PERVASIVE PLAYERS
- 11.7.4 PARTICIPANTS
- 11.7.5 COMPETITIVE BENCHMARKING: KEY PLAYERS, 2024
- 11.7.5.1 Company footprint
- 11.7.5.2 Region footprint
- 11.7.5.3 Product footprint
- 11.7.5.4 Functionality footprint
- 11.7.5.5 Functionality application footprint
- 11.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 11.8.1 PROGRESSIVE COMPANIES
- 11.8.2 RESPONSIVE COMPANIES
- 11.8.3 DYNAMIC COMPANIES
- 11.8.4 STARTING BLOCKS
- 11.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 11.8.5.1 Detailed list of key startups/SMEs
- 11.8.5.2 Competitive benchmarking of key startups/SMEs
- 11.9 COMPETITIVE SCENARIO
- 11.9.1 PRODUCT LAUNCHES AND APPROVALS
- 11.9.2 DEALS
- 11.9.3 EXPANSIONS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- 12.1.1 INTERNATIONAL FLAVORS & FRAGRANCES INC.
- 12.1.1.1 Business overview
- 12.1.1.2 Products offered
- 12.1.1.3 Recent developments
- 12.1.1.3.1 Deals
- 12.1.1.3.2 Expansions
- 12.1.1.4 MnM view
- 12.1.1.4.1 Key strengths
- 12.1.1.4.2 Strategic choices
- 12.1.1.4.3 Weaknesses and competitive threats
- 12.1.2 ADM
- 12.1.2.1 Business overview
- 12.1.2.2 Products offered
- 12.1.2.3 Recent developments
- 12.1.2.3.1 Deals
- 12.1.2.4 MnM view
- 12.1.2.4.1 Key strengths
- 12.1.2.4.2 Strategic choices
- 12.1.2.4.3 Weaknesses and competitive threats
- 12.1.3 ROQUETTE FRERES
- 12.1.3.1 Business overview
- 12.1.3.2 Products offered
- 12.1.3.3 Recent developments
- 12.1.3.3.1 Product launches and approvals
- 12.1.3.4 MnM view
- 12.1.3.4.1 Key strengths
- 12.1.3.4.2 Strategic choices
- 12.1.3.4.3 Weaknesses and competitive threats
- 12.1.4 BASF SE
- 12.1.4.1 Business overview
- 12.1.4.2 Products offered
- 12.1.4.3 Recent developments
- 12.1.4.3.1 Deals
- 12.1.4.3.2 Expansions
- 12.1.4.4 MnM view
- 12.1.4.4.1 Key strengths
- 12.1.4.4.2 Strategic choices
- 12.1.4.4.3 Weaknesses and competitive threats
- 12.1.5 EVONIK
- 12.1.5.1 Business overview
- 12.1.5.2 Products offered
- 12.1.5.3 Recent developments
- 12.1.5.3.1 Expansions
- 12.1.5.4 MnM view
- 12.1.5.4.1 Key strengths
- 12.1.5.4.2 Strategic choices
- 12.1.5.4.3 Weaknesses and competitive threats
- 12.1.6 ASHLAND
- 12.1.6.1 Business overview
- 12.1.6.2 Products offered
- 12.1.6.3 Recent developments
- 12.1.6.3.1 Product launches and approvals
- 12.1.7 KERRY GROUP PLC
- 12.1.7.1 Business overview
- 12.1.7.2 Products offered
- 12.1.7.3 Recent developments
- 12.1.7.3.1 Deals
- 12.1.8 MERCK
- 12.1.8.1 Business overview
- 12.1.8.2 Products offered
- 12.1.8.3 Recent developments
- 12.1.8.3.1 Deals
- 12.1.9 ASSOCIATED BRITISH FOODS PLC
- 12.1.9.1 Business overview
- 12.1.9.2 Products offered
- 12.1.9.3 Recent developments
- 12.1.9.3.1 Deals
- 12.1.10 WACKER CHEMIE AG
- 12.1.10.1 Business overview
- 12.1.10.2 Products offered
- 12.1.11 AIR LIQUIDE
- 12.1.11.1 Business overview
- 12.1.11.2 Products offered
- 12.1.11.3 Recent developments
- 12.1.11.3.1 Product launches and approvals
- 12.1.12 DOW
- 12.1.12.1 Business overview
- 12.1.12.2 Products offered
- 12.1.13 BERKSHIRE HATHAWAY (LUBRIZOL CORPORATION)
- 12.1.13.1 Business overview
- 12.1.13.2 Products offered
- 12.1.13.3 Recent developments
- 12.1.13.3.1 Product launches and approvals
- 12.1.14 COLORCON, INC.
- 12.1.14.1 Business overview
- 12.1.14.2 Products offered
- 12.1.14.3 Recent developments
- 12.1.14.3.1 Product launches and approvals
- 12.1.14.3.2 Deals
- 12.1.14.3.3 Expansions
- 12.1.15 DFE PHARMA
- 12.1.15.1 Business overview
- 12.1.15.2 Products offered
- 12.1.15.3 Recent developments
- 12.1.15.3.1 Deals
- 12.1.16 ACTYLIS
- 12.1.16.1 Business overview
- 12.1.16.2 Products offered
- 12.1.16.3 Recent developments
- 12.1.16.3.1 Deals
- 12.1.17 CRODA INTERNATIONAL PLC
- 12.1.17.1 Business overview
- 12.1.17.2 Products offered
- 12.1.17.3 Recent developments
- 12.1.17.3.1 Deals
- 12.1.17.3.2 Expansions
- 12.1.18 CHEMIE TRADE
- 12.1.18.1 Business overview
- 12.1.18.2 Products offered
- 12.1.19 GATTEFOSSE
- 12.1.19.1 Business overview
- 12.1.19.2 Products offered
- 12.1.20 J. RETTENMAIER & SOHNE GMBH + CO KG
- 12.1.20.1 Business overview
- 12.1.20.2 Products offered
- 12.1.21 SHIN-ETSU CHEMICAL CO., LTD.
- 12.1.21.1 Business overview
- 12.1.21.2 Products offered
- 12.1.21.3 Recent developments
- 12.1.21.3.1 Deals
- 12.1.21.3.2 Expansions
- 12.1.1 INTERNATIONAL FLAVORS & FRAGRANCES INC.
- 12.2 OTHER PLAYERS
- 12.2.1 INNOPHOS
- 12.2.2 MEGGLE GMBH & CO. KG
- 12.2.3 FUJI CHEMICAL INDUSTRIES CO., LTD.
- 12.2.4 COREL PHARMA CHEM
- 12.2.5 BIOGRUND
- 12.2.6 NITIKA PHARMACEUTICAL SPECIALTIES PVT. LTD.
- 12.2.7 R.T. VANDERBILT HOLDING COMPANY, INC.
- 12.2.8 BENEO
- 12.2.9 SIAGACHI INDUSTRIES
- 12.2.10 JH NANHANG LIFE SCIENCES CO., LTD.
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 CUSTOMIZATION OPTIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS
List of Tables
- TABLE 1 PHARMACEUTICAL EXCIPIENTS MARKET: INCLUSIONS AND EXCLUSIONS
- TABLE 2 IMPACT ANALYSIS OF SUPPLY-SIDE AND DEMAND-SIDE FACTORS
- TABLE 3 PHARMACEUTICAL EXCIPIENTS MARKET: RISK ANALYSIS
- TABLE 4 PHARMACEUTICAL EXCIPIENTS MARKET: IMPACT ANALYSIS OF MARKET DYNAMICS
- TABLE 5 IMPENDING AND ONGOING PATENT EXPIRY OF BLOCKBUSTER THERAPEUTICS, 2023-2035
- TABLE 6 LIST OF MULTIFUNCTIONAL EXCIPIENTS
- TABLE 7 INDICATIVE PRICING ANALYSIS OF EXCIPIENT PRODUCTS, BY KEY PLAYER, 2024
- TABLE 8 INDICATIVE PRICING ANALYSIS OF EXCIPIENT PRODUCTS, BY REGION, 2024
- TABLE 9 PHARMACEUTICAL EXCIPIENTS MARKET: ROLE OF COMPANIES IN ECOSYSTEM
- TABLE 10 PHARMACEUTICAL EXCIPIENTS MARKET: NUMBER OF PATENTS FILED, 2014-2024
- TABLE 11 INNOVATIONS AND PATENT REGISTRATIONS, 2022-2023
- TABLE 12 IMPORT DATA FOR HS CODE 290545 (GLYCEROL), 2020-2024 (USD)
- TABLE 13 IMPORT DATA FOR HS CODE 290545 (GLYCEROL), 2020-2024 (TONS)
- TABLE 14 EXPORT DATA FOR HS CODE 290545 (GLYCEROL), 2020-2024 (USD)
- TABLE 15 EXPORT DATA FOR HS CODE 290545 (GLYCEROL), 2020-2024 (TONS)
- TABLE 16 IMPORT DATA FOR HS CODE 350510 (DEXTRINS AND OTHER MODIFIED STARCHES, E.G. PREGELATINISED OR ESTERIFIED STARCHES), 2020-2024 (USD)
- TABLE 17 IMPORT DATA FOR HS CODE 350510 (DEXTRINS AND OTHER MODIFIED STARCHES, E.G. PREGELATINISED OR ESTERIFIED STARCHES), 2020-2024 (TONS)
- TABLE 18 EXPORT DATA FOR HS CODE 350510 (DEXTRINS AND OTHER MODIFIED STARCHES, E.G. PREGELATINISED OR ESTERIFIED STARCHES), 2020-2024 (USD)
- TABLE 19 EXPORT DATA FOR HS CODE 350510 (DEXTRINS AND OTHER MODIFIED STARCHES, E.G. PREGELATINISED OR ESTERIFIED STARCHES), 2020-2024 (TONS)
- TABLE 20 IMPORT DATA FOR HS CODE 290532 (PROPYLENE GLYCOL "PROPANE-1,2-DIOL), 2020-2024 (USD)
- TABLE 21 IMPORT DATA FOR HS CODE 290532 (PROPYLENE GLYCOL "PROPANE-1,2-DIOL), 2020-2024 (TONS)
- TABLE 22 EXPORT DATA FOR HS CODE 290532 (PROPYLENE GLYCOL "PROPANE-1,2-DIOL), 2020-2024 (USD)
- TABLE 23 EXPORT DATA FOR HS CODE 290532 (PROPYLENE GLYCOL "PROPANE-1,2-DIOL), 2020-2024 (TONS)
- TABLE 24 IMPORT DATA FOR HS CODE 283650 (CALCIUM CARBONATE), 2020-2024 (USD)
- TABLE 25 IMPORT DATA FOR HS CODE 283650 (CALCIUM CARBONATE), 2020-2024 (TONS)
- TABLE 26 EXPORT DATA FOR HS CODE 283650 (CALCIUM CARBONATE), 2020-2024 (USD)
- TABLE 27 EXPORT DATA FOR HS CODE 283650 (CALCIUM CARBONATE), 2020-2024 (TONS)
- TABLE 28 IMPORT DATA FOR HS CODE 250100 (SALTS, INCL. TABLE SALT AND DENATURED SALT, AND PURE SODIUM CHLORIDE), 2020-2024 (USD)
- TABLE 29 IMPORT DATA FOR HS CODE 250100 (SALTS, INCL. TABLE SALT AND DENATURED SALT, AND PURE SODIUM CHLORIDE), 2020-2024 (TONS)
- TABLE 30 EXPORT DATA FOR HS CODE 250100 (SALTS, INCL. TABLE SALT AND DENATURED SALT, AND PURE SODIUM CHLORIDE), 2020-2024 (USD)
- TABLE 31 EXPORT DATA FOR HS CODE 250100 (SALTS, INCL. TABLE SALT AND DENATURED SALT, AND PURE SODIUM CHLORIDE), 2020-2024 (TONS)
- TABLE 32 IMPORT DATA FOR HS CODE 290543 (MANNITOL), 2020-2024 (USD)
- TABLE 33 IMPORT DATA FOR HS CODE 290543 (MANNITOL), 2020-2024 (TONS)
- TABLE 34 EXPORT DATA FOR HS CODE 290543 (MANNITOL), 2020-2024 (USD)
- TABLE 35 EXPORT DATA FOR HS CODE 290543 (MANNITOL), 2020-2024 (TONS)
- TABLE 36 IMPORT DATA FOR HS CODE: 3912 (CELLULOSE AND ITS CHEMICAL DERIVATIVES, N.E.S., IN PRIMARY FORMS), 2020-2024 (USD)
- TABLE 37 IMPORT DATA FOR HS CODE: 3912 (CELLULOSE AND ITS CHEMICAL DERIVATIVES, N.E.S., IN PRIMARY FORMS), 2020-2024 (TONS)
- TABLE 38 EXPORT DATA FOR HS CODE: 3912 (CELLULOSE AND ITS CHEMICAL DERIVATIVES, N.E.S., IN PRIMARY FORMS), 2020-2024 (USD)
- TABLE 39 EXPORT DATA FOR HS CODE: 3912 (CELLULOSE AND ITS CHEMICAL DERIVATIVES, N.E.S., IN PRIMARY FORMS), 2020-2024 (TONS)
- TABLE 40 PHARMACEUTICAL EXCIPIENTS MARKET: KEY CONFERENCES AND EVENTS, 2025-2026
- TABLE 41 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 42 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 43 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 44 LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 45 MIDDLE EAST & AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 46 PHARMACEUTICAL EXCIPIENTS MARKET: PORTER'S FIVE FORCES ANALYSIS
- TABLE 47 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT (%)
- TABLE 48 KEY BUYING CRITERIA, BY END USER
- TABLE 49 US ADJUSTED RECIPROCAL TARIFF RATES
- TABLE 50 COMPARATIVE PRICING ON KEY EXCIPIENT PRE AND POST US 2025 TARIFF
- TABLE 51 IMPACT ANALYSIS ON END-USE INDUSTRIES
- TABLE 52 PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 53 PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 54 PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 55 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 56 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 57 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 58 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 59 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 60 PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 61 PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 62 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 63 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 64 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 65 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 66 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 67 PHARMACEUTICAL EXCIPIENTS MARKET FOR FATTY ALCOHOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 68 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR FATTY ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 69 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR FATTY ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 70 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR FATTY ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 71 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR FATTY ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 72 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR FATTY ALCOHOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 73 PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL STEARATES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 74 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL STEARATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 75 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL STEARATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 76 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL STEARATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 77 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL STEARATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 78 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL STEARATES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 79 PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCERIN, BY REGION, 2023-2030 (USD MILLION)
- TABLE 80 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCERIN, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 81 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCERIN, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 82 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCERIN, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 83 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCERIN, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 84 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCERIN, BY REGION, 2023-2030 (USD MILLION)
- TABLE 85 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER OLEOCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 86 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 87 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 88 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 89 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER OLEOCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 90 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER OLEOCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 91 PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 92 PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 93 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 94 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 95 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 96 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 97 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 98 PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 99 PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 100 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 101 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 102 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 103 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 104 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 105 PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 106 PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 107 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 108 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 109 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 110 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 111 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 112 PHARMACEUTICAL EXCIPIENTS MARKET FOR LACTOSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 113 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR LACTOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 114 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR LACTOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 115 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR LACTOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 116 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR LACTOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 117 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR LACTOSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 118 PHARMACEUTICAL EXCIPIENTS MARKET FOR SUCROSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 119 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUCROSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 120 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUCROSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 121 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUCROSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 122 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUCROSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 123 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUCROSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 124 PHARMACEUTICAL EXCIPIENTS MARKET FOR DEXTROSE (D-GLUCOSE), BY REGION, 2023-2030 (USD MILLION)
- TABLE 125 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR DEXTROSE (D-GLUCOSE), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 126 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR DEXTROSE (D-GLUCOSE), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 127 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR DEXTROSE (D-GLUCOSE), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 128 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR DEXTROSE (D-GLUCOSE), BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 129 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR DEXTROSE (D-GLUCOSE), BY REGION, 2023-2030 (USD MILLION)
- TABLE 130 PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 131 PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 132 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 133 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 134 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 135 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 136 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 137 PHARMACEUTICAL EXCIPIENTS MARKET FOR SORBITOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 138 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SORBITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 139 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SORBITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 140 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SORBITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 141 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SORBITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 142 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SORBITOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 143 PHARMACEUTICAL EXCIPIENTS MARKET FOR MANNITOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 144 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MANNITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 145 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MANNITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 146 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MANNITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 147 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MANNITOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 148 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MANNITOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 149 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER SUGAR ALCOHOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 150 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 151 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 152 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 153 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER SUGAR ALCOHOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 154 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER SUGAR ALCOHOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 155 PHARMACEUTICAL EXCIPIENTS MARKET FOR ARTIFICIAL SWEETENERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 156 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ARTIFICIAL SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 157 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ARTIFICIAL SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 158 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ARTIFICIAL SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 159 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ARTIFICIAL SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 160 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ARTIFICIAL SWEETENERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 161 PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 162 PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 163 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 164 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 165 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 166 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 167 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 168 PHARMACEUTICAL EXCIPIENTS MARKET FOR MICROCRYSTALLINE CELLULOSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 169 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MICROCRYSTALLINE CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 170 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MICROCRYSTALLINE CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 171 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MICROCRYSTALLINE CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 172 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MICROCRYSTALLINE CELLULOSE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 173 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MICROCRYSTALLINE CELLULOSE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 174 PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ETHERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 175 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ETHERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 176 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ETHERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 177 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ETHERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 178 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ETHERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 179 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ETHERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 180 PHARMACEUTICAL EXCIPIENTS MARKET FOR CMC & CROSCARMELLOSE SODIUM, BY REGION, 2023-2030 (USD MILLION)
- TABLE 181 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CMC & CROSCARMELLOSE SODIUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 182 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CMC & CROSCARMELLOSE SODIUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 183 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CMC & CROSCARMELLOSE SODIUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 184 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CMC & CROSCARMELLOSE SODIUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 185 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CMC & CROSCARMELLOSE SODIUM, BY REGION, 2023-2030 (USD MILLION)
- TABLE 186 PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ESTERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 187 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ESTERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 188 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ESTERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 189 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ESTERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 190 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ESTERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 191 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE ESTERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 192 PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 193 PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 194 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 195 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 196 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 197 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 198 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 199 PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 200 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 201 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 202 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 203 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 204 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 205 PHARMACEUTICAL EXCIPIENTS MARKET FOR DRIED STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 206 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR DRIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 207 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR DRIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 208 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR DRIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 209 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR DRIED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 210 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR DRIED STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 211 PHARMACEUTICAL EXCIPIENTS MARKET FOR CONVERTED STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 212 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CONVERTED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 213 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CONVERTED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 214 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CONVERTED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 215 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CONVERTED STARCH, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 216 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CONVERTED STARCH, BY REGION, 2023-2030 (USD MILLION)
- TABLE 217 PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 218 PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 219 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 220 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 221 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 222 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 223 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 224 PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 225 PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 226 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 227 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 228 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 229 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 230 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 231 PHARMACEUTICAL EXCIPIENTS MARKET FOR POLYETHYLENE GLYCOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 232 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR POLYETHYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 233 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR POLYETHYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 234 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR POLYETHYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 235 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR POLYETHYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 236 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR POLYETHYLENE GLYCOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 237 PHARMACEUTICAL EXCIPIENTS MARKET FOR PROPYLENE GLYCOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 238 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROPYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 239 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROPYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 240 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROPYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 241 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROPYLENE GLYCOL, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 242 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROPYLENE GLYCOL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 243 PHARMACEUTICAL EXCIPIENTS MARKET FOR POVIDONES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 244 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR POVIDONES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 245 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR POVIDONES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 246 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR POVIDONES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 247 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR POVIDONES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 248 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR POVIDONES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 249 PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 250 PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 251 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 252 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 253 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 254 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 255 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 256 PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROLATUM, BY REGION, 2023-2030 (USD MILLION)
- TABLE 257 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROLATUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 258 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROLATUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 259 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROLATUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 260 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROLATUM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 261 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROLATUM, BY REGION, 2023-2030 (USD MILLION)
- TABLE 262 PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL WAXES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 263 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL WAXES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 264 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL WAXES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 265 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL WAXES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 266 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL WAXES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 267 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL WAXES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 268 PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL OILS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 269 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL OILS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 270 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL OILS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 271 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL OILS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 272 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL OILS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 273 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL OILS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 274 PHARMACEUTICAL EXCIPIENTS MARKET FOR ACRYLIC POLYMERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 275 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACRYLIC POLYMERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 276 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACRYLIC POLYMERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 277 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACRYLIC POLYMERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 278 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACRYLIC POLYMERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 279 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACRYLIC POLYMERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 280 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER PETROCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 281 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 282 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 283 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 284 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER PETROCHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 285 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER PETROCHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 286 PHARMACEUTICAL EXCIPIENTS MARKET FOR PROTEINS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 287 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROTEINS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 288 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROTEINS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 289 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROTEINS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 290 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROTEINS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 291 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PROTEINS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 292 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 293 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 294 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 295 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 296 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 297 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 298 PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 299 PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 300 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 301 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 302 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 303 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 304 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 305 PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM PHOSPHATE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 306 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM PHOSPHATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 307 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM PHOSPHATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 308 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM PHOSPHATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 309 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM PHOSPHATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 310 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM PHOSPHATE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 311 PHARMACEUTICAL EXCIPIENTS MARKET FOR METAL OXIDES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 312 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR METAL OXIDES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 313 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR METAL OXIDES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 314 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR METAL OXIDES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 315 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR METAL OXIDES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 316 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR METAL OXIDES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 317 PHARMACEUTICAL EXCIPIENTS MARKET FOR HALITES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 318 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR HALITES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 319 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR HALITES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 320 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR HALITES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 321 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR HALITES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 322 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR HALITES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 323 PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM CARBONATE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 324 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM CARBONATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 325 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM CARBONATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 326 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM CARBONATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 327 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM CARBONATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 328 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM CARBONATE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 329 PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM SULFATE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 330 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM SULFATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 331 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM SULFATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 332 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM SULFATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 333 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM SULFATE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 334 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CALCIUM SULFATE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 335 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER INORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 336 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 337 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 338 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 339 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER INORGANIC CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 340 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER INORGANIC CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 341 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 342 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 343 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 344 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 345 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER CHEMICALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 346 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER CHEMICALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 347 PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 348 PHARMACEUTICAL EXCIPIENTS MARKET FOR FILLERS & DILUENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 349 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR FILLERS & DILUENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 350 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR FILLERS & DILUENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 351 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR FILLERS & DILUENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 352 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR FILLERS & DILUENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 353 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR FILLERS & DILUENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 354 PHARMACEUTICAL EXCIPIENTS MARKET FOR SUSPENDING & VISCOSITY AGENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 355 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUSPENDING & VISCOSITY AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 356 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUSPENDING & VISCOSITY AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 357 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUSPENDING & VISCOSITY AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 358 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUSPENDING & VISCOSITY AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 359 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUSPENDING & VISCOSITY AGENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 360 PHARMACEUTICAL EXCIPIENTS MARKET FOR COATING AGENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 361 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR COATING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 362 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR COATING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 363 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR COATING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 364 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR COATING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 365 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR COATING AGENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 366 PHARMACEUTICAL EXCIPIENTS MARKET FOR BINDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 367 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR BINDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 368 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR BINDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 369 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR BINDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 370 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR BINDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 371 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR BINDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 372 PHARMACEUTICAL EXCIPIENTS MARKET FOR FLAVORING AGENTS & SWEETENERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 373 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR FLAVORING AGENTS & SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 374 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR FLAVORING AGENTS & SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 375 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR FLAVORING AGENTS & SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 376 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR FLAVORING AGENTS & SWEETENERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 377 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR FLAVORING AGENTS & SWEETENERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 378 PHARMACEUTICAL EXCIPIENTS MARKET FOR DISINTEGRANTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 379 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR DISINTEGRANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 380 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR DISINTEGRANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 381 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR DISINTEGRANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 382 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR DISINTEGRANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 383 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR DISINTEGRANTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 384 PHARMACEUTICAL EXCIPIENTS MARKET FOR COLORANTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 385 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR COLORANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 386 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR COLORANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 387 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR COLORANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 388 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR COLORANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 389 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR COLORANTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 390 PHARMACEUTICAL EXCIPIENTS MARKET FOR LUBRICANTS & GLIDANTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 391 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR LUBRICANTS & GLIDANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 392 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR LUBRICANTS & GLIDANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 393 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR LUBRICANTS & GLIDANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 394 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR LUBRICANTS & GLIDANTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 395 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR LUBRICANTS & GLIDANTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 396 PHARMACEUTICAL EXCIPIENTS MARKET FOR PRESERVATIVES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 397 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PRESERVATIVES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 398 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PRESERVATIVES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 399 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PRESERVATIVES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 400 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PRESERVATIVES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 401 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PRESERVATIVES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 402 PHARMACEUTICAL EXCIPIENTS MARKET FOR EMULSIFYING AGENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 403 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR EMULSIFYING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 404 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR EMULSIFYING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 405 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR EMULSIFYING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 406 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR EMULSIFYING AGENTS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 407 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR EMULSIFYING AGENTS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 408 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FUNCTIONALITIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 409 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FUNCTIONALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 410 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FUNCTIONALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 411 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FUNCTIONALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 412 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FUNCTIONALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 413 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FUNCTIONALITIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 414 PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 415 PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 416 PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 417 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 418 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 419 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 420 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 421 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 422 TYPICAL COMPOSITION OF TABLETS
- TABLE 423 PHARMACEUTICAL EXCIPIENTS MARKET FOR TABLETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 424 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR TABLETS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 425 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR TABLETS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 426 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR TABLETS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 427 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR TABLETS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 428 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR TABLETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 429 PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 430 PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 431 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 432 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 433 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 434 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 435 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 436 PHARMACEUTICAL EXCIPIENTS MARKET FOR HARD-GELATIN CAPSULES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 437 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR HARD-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 438 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR HARD-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 439 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR HARD-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 440 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR HARD-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 441 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR HARD-GELATIN CAPSULES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 442 PHARMACEUTICAL EXCIPIENTS MARKET FOR SOFT-GELATIN CAPSULES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 443 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOFT-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 444 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOFT-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 445 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOFT-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 446 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOFT-GELATIN CAPSULES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 447 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOFT-GELATIN CAPSULES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 448 PHARMACEUTICAL EXCIPIENTS MARKET FOR LIQUID FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 449 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR LIQUID FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 450 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR LIQUID FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 451 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR LIQUID FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 452 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR LIQUID FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 453 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR LIQUID FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 454 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 455 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 456 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 457 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 458 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 459 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER ORAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 460 PHARMACEUTICAL EXCIPIENTS MARKET FOR TOPICAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 461 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR TOPICAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 462 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR TOPICAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 463 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR TOPICAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 464 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR TOPICAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 465 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR TOPICAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 466 TYPICAL COMPOSITION OF PARENTERAL FORMULATIONS
- TABLE 467 EXCIPIENTS USED IN PARENTERAL FORMULATIONS
- TABLE 468 PHARMACEUTICAL EXCIPIENTS MARKET FOR PARENTERAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 469 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PARENTERAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 470 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PARENTERAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 471 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PARENTERAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 472 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PARENTERAL FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 473 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PARENTERAL FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 474 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 475 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 476 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 477 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 478 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FORMULATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 479 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER FORMULATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 480 PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 481 PHARMACEUTICAL EXCIPIENTS MARKET FOR STABILIZERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 482 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STABILIZERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 483 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR STABILIZERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 484 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR STABILIZERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 485 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STABILIZERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 486 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR STABILIZERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 487 PHARMACEUTICAL EXCIPIENTS MARKET FOR TASTE MASKING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 488 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR TASTE MASKING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 489 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR TASTE MASKING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 490 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR TASTE MASKING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 491 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR TASTE MASKING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 492 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR TASTE MASKING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 493 PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED-RELEASE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 494 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED-RELEASE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 495 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED-RELEASE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 496 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED-RELEASE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 497 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED-RELEASE, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 498 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MODIFIED-RELEASE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 499 PHARMACEUTICAL EXCIPIENTS MARKET FOR SOLUBILITY & BIOAVAILABILITY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 500 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOLUBILITY & BIOAVAILABILITY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 501 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOLUBILITY & BIOAVAILABILITY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 502 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOLUBILITY & BIOAVAILABILITY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 503 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOLUBILITY & BIOAVAILABILITY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 504 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SOLUBILITY & BIOAVAILABILITY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 505 PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 506 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 507 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 508 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 509 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 510 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OTHER APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 511 PHARMACEUTICAL EXCIPIENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 512 EUROPE: KEY MACROECONOMIC INDICATORS
- TABLE 513 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 514 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 515 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 516 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 517 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 518 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 519 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 520 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 521 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 522 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 523 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 524 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 525 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 526 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 527 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 528 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 529 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 530 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 531 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 532 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 533 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 534 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 535 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 536 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 537 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 538 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 539 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 540 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 541 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 542 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 543 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 544 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 545 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 546 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 547 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 548 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 549 GERMANY: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 550 UK: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 551 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 552 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 553 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 554 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 555 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 556 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 557 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 558 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 559 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 560 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 561 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 562 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 563 UK: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 564 UK: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 565 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 566 UK: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 567 UK: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 568 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 569 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 570 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 571 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 572 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 573 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 574 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 575 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 576 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 577 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 578 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 579 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 580 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 581 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 582 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 583 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 584 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 585 FRANCE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 586 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 587 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 588 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 589 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 590 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 591 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 592 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 593 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 594 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 595 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 596 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 597 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 598 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 599 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 600 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 601 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 602 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 603 ITALY: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 604 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 605 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 606 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 607 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 608 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 609 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 610 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 611 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 612 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 613 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 614 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 615 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 616 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 617 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 618 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 619 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 620 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 621 SPAIN: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 622 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 623 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 624 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 625 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 626 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 627 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 628 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 629 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 630 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 631 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 632 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 633 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 634 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 635 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 636 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 637 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 638 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 639 REST OF EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 640 NORTH AMERICA: KEY MACROECONOMIC INDICATORS
- TABLE 641 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 642 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 643 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 644 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 645 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 646 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 647 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 648 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 649 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 650 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 651 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 652 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 653 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 654 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 655 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 656 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 657 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 658 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 659 NORTH AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 660 US: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 661 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 662 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 663 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 664 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 665 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 666 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 667 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 668 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 669 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 670 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 671 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 672 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 673 US: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 674 US: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 675 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 676 US: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 677 US: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 678 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 679 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 680 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 681 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 682 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 683 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 684 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 685 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 686 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 687 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 688 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 689 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 690 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 691 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 692 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 693 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 694 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 695 CANADA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 696 ASIA PACIFIC: KEY MACROECONOMIC INDICATORS
- TABLE 697 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 698 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 699 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 700 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 701 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 702 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 703 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 704 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 705 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 706 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 707 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 708 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 709 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 710 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 711 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 712 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 713 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 714 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 715 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 716 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 717 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 718 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 719 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 720 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 721 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 722 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 723 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 724 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 725 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 726 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 727 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 728 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 729 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 730 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 731 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 732 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 733 CHINA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 734 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 735 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 736 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 737 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 738 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 739 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 740 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 741 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 742 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 743 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 744 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 745 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 746 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 747 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 748 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 749 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 750 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 751 JAPAN: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 752 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 753 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 754 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 755 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 756 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 757 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 758 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 759 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 760 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 761 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 762 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 763 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 764 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 765 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 766 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 767 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 768 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 769 INDIA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 770 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 771 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 772 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 773 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 774 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 775 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 776 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 777 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 778 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 779 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 780 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 781 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 782 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 783 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 784 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 785 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 786 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 787 SOUTH KOREA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 788 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 789 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 790 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 791 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 792 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 793 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 794 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 795 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 796 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 797 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 798 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 799 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 800 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 801 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 802 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 803 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 804 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 805 REST OF ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 806 LATIN AMERICA: KEY MACROECONOMIC INDICATORS
- TABLE 807 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 808 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 809 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 810 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 811 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 812 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 813 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 814 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 815 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 816 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 817 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 818 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 819 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 820 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 821 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 822 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 823 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 824 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 825 LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 826 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 827 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 828 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 829 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 830 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 831 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 832 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 833 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 834 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 835 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 836 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 837 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 838 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 839 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 840 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 841 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 842 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 843 BRAZIL: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 844 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 845 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 846 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 847 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 848 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 849 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 850 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 851 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 852 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 853 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 854 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 855 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 856 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 857 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 858 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 859 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 860 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 861 MEXICO: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 862 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 863 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 864 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 865 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 866 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 867 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 868 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 869 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 870 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 871 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 872 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 873 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 874 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 875 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 876 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 877 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 878 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 879 REST OF LATIN AMERICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 880 MIDDLE EAST: KEY MACROECONOMIC INDICATORS
- TABLE 881 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 882 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 883 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 884 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 885 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 886 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 887 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 888 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 889 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 890 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 891 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 892 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 893 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 894 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 895 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 896 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 897 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 898 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 899 MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 900 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 901 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 902 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 903 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 904 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 905 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 906 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 907 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 908 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 909 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 910 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 911 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 912 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 913 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 914 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 915 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 916 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 917 GCC COUNTRIES: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 918 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 919 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 920 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 921 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 922 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 923 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 924 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 925 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 926 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 927 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 928 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 929 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 930 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 931 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 932 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 933 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 934 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 935 REST OF MIDDLE EAST: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 936 AFRICA: KEY MACROECONOMIC INDICATORS
- TABLE 937 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2023-2030 (USD MILLION)
- TABLE 938 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 939 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR OLEOCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 940 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CARBOHYDRATES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 941 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 942 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ACTUAL SUGARS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 943 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR SUGAR ALCOHOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 944 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CELLULOSE, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 945 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR STARCH, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 946 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR PETROCHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 947 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR GLYCOLS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 948 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR MINERAL HYDROCARBONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 949 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR INORGANIC CHEMICALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 950 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2023-2030 (USD MILLION)
- TABLE 951 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2023-2030 (USD MILLION)
- TABLE 952 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR ORAL FORMULATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 953 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET FOR CAPSULES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 954 AFRICA: PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 955 OVERVIEW OF MAJOR STRATEGIES DEPLOYED BY KEY PLAYERS IN PHARMACEUTICAL EXCIPIENTS MARKET, 2022-2024
- TABLE 956 PHARMACEUTICAL EXCIPIENTS MARKET: DEGREE OF COMPETITION
- TABLE 957 PHARMACEUTICAL EXCIPIENTS MARKET: REGION FOOTPRINT
- TABLE 958 PHARMACEUTICAL EXCIPIENTS MARKET: PRODUCT FOOTPRINT
- TABLE 959 PHARMACEUTICAL EXCIPIENTS MARKET: FUNCTIONALITY FOOTPRINT
- TABLE 960 PHARMACEUTICAL EXCIPIENTS MARKET: FUNCTIONALITY APPLICATION FOOTPRINT
- TABLE 961 PHARMACEUTICAL EXCIPIENTS MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 962 PHARMACEUTICAL EXCIPIENTS MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES, 2024
- TABLE 963 PHARMACEUTICAL EXCIPIENTS MARKET: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 964 PHARMACEUTICAL EXCIPIENTS MARKET: DEALS, JANUARY 2022-JUNE 2025
- TABLE 965 PHARMACEUTICAL EXCIPIENTS MARKET: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 966 INTERNATIONAL FLAVORS & FRAGRANCES INC.: COMPANY OVERVIEW
- TABLE 967 INTERNATIONAL FLAVORS & FRAGRANCES INC.: PRODUCTS OFFERED
- TABLE 968 INTERNATIONAL FLAVORS & FRAGRANCES INC.: DEALS, JANUARY 2022-JUNE 2025
- TABLE 969 INTERNATIONAL FLAVORS & FRAGRANCES INC.: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 970 ADM: COMPANY OVERVIEW
- TABLE 971 ADM: PRODUCTS OFFERED
- TABLE 972 ADM: DEALS, JANUARY 2022-JUNE 2025
- TABLE 973 ROQUETTE FRERES: COMPANY OVERVIEW
- TABLE 974 ROQUETTE FRERES: PRODUCTS OFFERED
- TABLE 975 ROQUETTE FRERES: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 976 ROQUETTE FRERES: DEALS, JANUARY 2022-JUNE 2025
- TABLE 977 BASF SE: COMPANY OVERVIEW
- TABLE 978 BASF SE: PRODUCTS OFFERED
- TABLE 979 BASF SE: DEALS, JANUARY 2022-JUNE 2025
- TABLE 980 BASF SE: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 981 EVONIK: COMPANY OVERVIEW
- TABLE 982 EVONIK: PRODUCTS OFFERED
- TABLE 983 EVONIK: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 984 ASHLAND: COMPANY OVERVIEW
- TABLE 985 ASHLAND: PRODUCTS OFFERED
- TABLE 986 ASHLAND: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 987 KERRY GROUP PLC: COMPANY OVERVIEW
- TABLE 988 KERRY GROUP PLC: PRODUCTS OFFERED
- TABLE 989 KERRY GROUP PLC: DEALS, JANUARY 2022-JUNE 2025
- TABLE 990 MERCK: COMPANY OVERVIEW
- TABLE 991 MERCK: PRODUCTS OFFERED
- TABLE 992 MERCK: DEALS, JANUARY 2022-JUNE 2025
- TABLE 993 ASSOCIATED BRITISH FOODS PLC: COMPANY OVERVIEW
- TABLE 994 ASSOCIATED BRITISH FOODS PLC: PRODUCTS OFFERED
- TABLE 995 ASSOCIATED BRITISH FOODS PLC: DEALS, JANUARY 2022-JUNE 2025
- TABLE 996 WACKER CHEMIE AG: COMPANY OVERVIEW
- TABLE 997 WACKER CHEMIE AG: PRODUCTS OFFERED
- TABLE 998 AIR LIQUIDE: COMPANY OVERVIEW
- TABLE 999 AIR LIQUIDE: PRODUCTS OFFERED
- TABLE 1000 AIR LIQUIDE: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 1001 DOW: COMPANY OVERVIEW
- TABLE 1002 DOW: PRODUCTS OFFERED
- TABLE 1003 BERKSHIRE HATHAWAY: COMPANY OVERVIEW
- TABLE 1004 BERKSHIRE HATHAWAY: PRODUCTS OFFERED
- TABLE 1005 BERKSHIRE HATHAWAY: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 1006 COLORCON, INC.: COMPANY OVERVIEW
- TABLE 1007 COLORCON, INC.: PRODUCTS OFFERED
- TABLE 1008 COLORCON, INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 1009 COLORCON, INC.: DEALS, JANUARY 2022-JUNE 2025
- TABLE 1010 COLORCON, INC.: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 1011 DFE PHARMA: COMPANY OVERVIEW
- TABLE 1012 DFE PHARMA: PRODUCTS OFFERED
- TABLE 1013 DFE PHARMA: DEALS, JANUARY 2022-JUNE 2025
- TABLE 1014 ACTYLIS: COMPANY OVERVIEW
- TABLE 1015 ACTYLIS: PRODUCTS OFFERED
- TABLE 1016 ACTYLIS: DEALS, JANUARY 2022-JUNE 2025
- TABLE 1017 CRODA INTERNATIONAL PLC: COMPANY OVERVIEW
- TABLE 1018 CRODA INTERNATIONAL PLC: PRODUCTS OFFERED
- TABLE 1019 CRODA INTERNATIONAL PLC: DEALS, JANUARY 2022-JUNE 2025
- TABLE 1020 CRODA INTERNATIONAL PLC: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 1021 CHEMIE TRADE: COMPANY OVERVIEW
- TABLE 1022 CHEMIE TRADE: PRODUCTS OFFERED
- TABLE 1023 GATTEFOSSE: COMPANY OVERVIEW
- TABLE 1024 GATTEFOSSE: PRODUCTS OFFERED
- TABLE 1025 J. RETTENMAIER & SOHNE GMBH + CO KG: COMPANY OVERVIEW
- TABLE 1026 J. RETTENMAIER & SOHNE GMBH + CO KG: PRODUCTS OFFERED
- TABLE 1027 SHIN-ETSU CHEMICAL CO., LTD.: COMPANY OVERVIEW
- TABLE 1028 SHIN-ETSU CHEMICAL CO., LTD.: PRODUCTS OFFERED
- TABLE 1029 SHIN-ETSU CHEMICAL CO., LTD.: DEALS, JANUARY 2022-JUNE 2025
- TABLE 1030 SHIN-ETSU CHEMICAL CO., LTD.: EXPANSIONS, JANUARY 2022-JUNE 2025
- TABLE 1031 INNOPHOS: COMPANY OVERVIEW
- TABLE 1032 MEGGLE GMBH & CO. KG: COMPANY OVERVIEW
- TABLE 1033 FUJI CHEMICAL INDUSTRIES CO., LTD.: COMPANY OVERVIEW
- TABLE 1034 COREL PHARMA CHEM: COMPANY OVERVIEW
- TABLE 1035 BIOGRUND: COMPANY OVERVIEW
- TABLE 1036 NITIKA PHARMACEUTICAL SPECIALTIES PVT. LTD.: COMPANY OVERVIEW
- TABLE 1037 R.T. VANDERBILT HOLDING COMPANY, INC.: COMPANY OVERVIEW
- TABLE 1038 BENEO: COMPANY OVERVIEW
- TABLE 1039 SIAGACHI INDUSTRIES: COMPANY OVERVIEW
- TABLE 1040 JH NANHANG LIFE SCIENCES CO., LTD.: COMPANY OVERVIEW
List of Figures
- FIGURE 1 PHARMACEUTICAL EXCIPIENTS MARKET SEGMENTATION
- FIGURE 2 PHARMACEUTICAL EXCIPIENTS REGIONAL SCOPE
- FIGURE 3 PHARMACEUTICAL EXCIPIENTS MARKET: YEARS CONSIDERED
- FIGURE 4 PHARMACEUTICAL EXCIPIENTS MARKET: RESEARCH DESIGN
- FIGURE 5 PHARMACEUTICAL EXCIPIENTS MARKET: KEY DATA FROM SECONDARY SOURCES
- FIGURE 6 PHARMACEUTICAL EXCIPIENTS MARKET: BREAKDOWN OF PRIMARY INTERVIEWS
- FIGURE 7 PHARMACEUTICAL EXCIPIENTS MARKET ESTIMATION (SUPPLY-SIDE ANALYSIS), 2024
- FIGURE 8 COMPANY REVENUE ANALYSIS-BASED ESTIMATION: BOTTOM-UP APPROACH, 2024
- FIGURE 9 REVENUE SHARE ANALYSIS OF SANDOZ GROUP AG, 2024
- FIGURE 10 PHARMACEUTICAL EXCIPIENTS MARKET SIZE VALIDATION FROM PRIMARY SOURCES
- FIGURE 11 PHARMACEUTICAL EXCIPIENTS MARKET: TOP-DOWN APPROACH
- FIGURE 12 PHARMACEUTICAL EXCIPIENTS MARKET: CAGR PROJECTIONS
- FIGURE 13 PHARMACEUTICAL EXCIPIENTS MARKET: DATA TRIANGULATION
- FIGURE 14 PHARMACEUTICAL EXCIPIENTS MARKET, BY PRODUCT, 2025 VS. 2030 (USD MILLION)
- FIGURE 15 PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY, 2025 VS. 2030 (USD MILLION)
- FIGURE 16 PHARMACEUTICAL EXCIPIENTS MARKET, BY FORMULATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 17 PHARMACEUTICAL EXCIPIENTS MARKET, BY FUNCTIONALITY APPLICATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 18 GEOGRAPHICAL SNAPSHOT OF PHARMACEUTICAL EXCIPIENTS MARKET
- FIGURE 19 GROWING DEMAND FOR GENERICS AND PHARMACEUTICAL PRODUCTS TO DRIVE MARKET
- FIGURE 20 ORGANIC CHEMICALS SEGMENT AND US TO LEAD NORTH AMERICAN MARKET IN 2025
- FIGURE 21 GERMANY TO REGISTER HIGHEST GROWTH DURING FORECAST PERIOD
- FIGURE 22 EMERGING MARKETS TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
- FIGURE 23 PHARMACEUTICAL EXCIPIENTS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 24 GLOBAL PHARMACEUTICAL R&D SPEND, 2018-2028
- FIGURE 25 REVENUE SHIFT AND NEW POCKETS FOR PHARMACEUTICAL EXCIPIENT PROVIDERS
- FIGURE 26 INDICATIVE PRICING ANALYSIS EXCIPIENT PRODUCTS, 2024
- FIGURE 27 INDICATIVE PRICING ANALYSIS OF EXCIPIENT PRODUCTS, BY REGION, 2024
- FIGURE 28 PHARMACEUTICAL EXCIPIENTS MARKET: VALUE CHAIN ANALYSIS
- FIGURE 29 PHARMACEUTICAL EXCIPIENTS MARKET: SUPPLY CHAIN ANALYSIS
- FIGURE 30 PHARMACEUTICAL EXCIPIENTS MARKET: ECOSYSTEM ANALYSIS
- FIGURE 31 PHARMACEUTICAL EXCIPIENTS MARKET: INVESTMENT AND FUNDING SCENARIO, 2022-2024
- FIGURE 32 PHARMACEUTICAL EXCIPIENTS MARKET: PATENT ANALYSIS, JANUARY 2014-DECEMBER 2024
- FIGURE 33 PHARMACEUTICAL EXCIPIENTS MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 34 INFLUENCE OF KEY STAKEHOLDERS IN BUYING PROCESS, BY PRODUCT
- FIGURE 35 KEY BUYING CRITERIA, BY END USER
- FIGURE 36 PHARMACEUTICAL EXCIPIENTS MARKET: AI USE CASES
- FIGURE 37 EUROPE: PHARMACEUTICAL EXCIPIENTS MARKET SNAPSHOT
- FIGURE 38 ASIA PACIFIC: PHARMACEUTICAL EXCIPIENTS MARKET SNAPSHOT
- FIGURE 39 REVENUE ANALYSIS OF KEY PLAYERS, 2020-2024 (USD MILLION)
- FIGURE 40 MARKET SHARE ANALYSIS OF KEY PLAYERS IN PHARMACEUTICAL EXCIPIENTS MARKET, 2024
- FIGURE 41 EV/EBITDA OF KEY VENDORS
- FIGURE 42 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 43 PHARMACEUTICAL EXCIPIENTS MARKET: BRAND/PRODUCT COMPARISON
- FIGURE 44 PHARMACEUTICAL EXCIPIENTS MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 45 PHARMACEUTICAL EXCIPIENTS MARKET: COMPANY FOOTPRINT
- FIGURE 46 PHARMACEUTICAL EXCIPIENTS MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 47 INTERNATIONAL FLAVORS & FRAGRANCES INC.: COMPANY SNAPSHOT (2024)
- FIGURE 48 ADM: COMPANY SNAPSHOT (2024)
- FIGURE 49 ROQUETTE FRERES: COMPANY SNAPSHOT (2024)
- FIGURE 50 BASF SE: COMPANY SNAPSHOT (2024)
- FIGURE 51 EVONIK: COMPANY SNAPSHOT (2024)
- FIGURE 52 ASHLAND: COMPANY SNAPSHOT (2024)
- FIGURE 53 KERRY GROUP PLC: COMPANY SNAPSHOT (2024)
- FIGURE 54 MERCK: COMPANY SNAPSHOT (2024)
- FIGURE 55 ASSOCIATED BRITISH FOODS PLC: COMPANY SNAPSHOT (2024)
- FIGURE 56 WACKER CHEMIE AG: COMPANY SNAPSHOT (2024)
- FIGURE 57 AIR LIQUIDE: COMPANY SNAPSHOT (2024)
- FIGURE 58 DOW: COMPANY SNAPSHOT (2024)
- FIGURE 59 BERKSHIRE HATHAWAY: COMPANY SNAPSHOT (2024)
- FIGURE 60 CRODA INTERNATIONAL PLC: COMPANY SNAPSHOT (2024)
- FIGURE 61 SHIN-ETSU CHEMICAL CO., LTD.: COMPANY SNAPSHOT (2024)











