 |
市場調查報告書
商品編碼
1913432
輔助生殖技術市場機會、成長要素、產業趨勢分析及2026年至2035年預測Assisted Reproductive Technology (ART) Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035 |
||||||
全球輔助生殖技術(ART)市場預計到 2025 年將達到 389 億美元,到 2035 年將達到 798 億美元,年複合成長率為 7.6%。
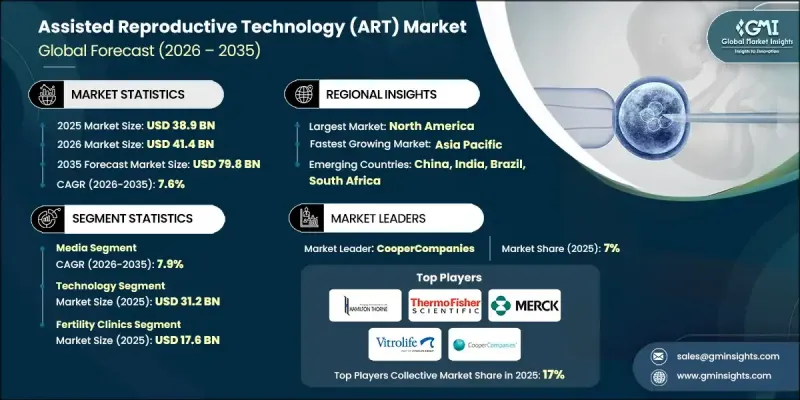
市場擴張的驅動力在於持續不斷的技術創新,這些創新不斷改善治療效果和患者體驗。人工智慧輔助胚胎選擇、延時成像和胚胎著床前遺傳學檢測 (PGT) 等技術創新提高了胚胎著床成功率,同時降低了週期失敗率,使輔助生殖技術 (ART) 更可靠、更具吸引力。輔助生殖技術 (ART) 是指旨在幫助自然受孕困難的個人和夫婦的醫療程序。它涉及在體外對卵子、精子和胚胎進行操作。社會規範的改變以及對包括單親家庭和同性伴侶家庭在內的多元化家庭結構的日益包容,正在推動對輔助生殖技術解決方案的需求。公眾意識的提高、父母期望的改變以及對替代性生殖方法的接受度不斷提高,正在加速市場成長,並為全球的診所、技術提供者和相關服務提供者創造機會。
| 市場覆蓋範圍 | |
|---|---|
| 開始年份 | 2025 |
| 預測年份 | 2026-2035 |
| 起始值 | 389億美元 |
| 預測金額 | 798億美元 |
| 複合年成長率 | 7.6% |
到 2025 年,技術細分市場將達到 312 億美元,這反映出由於生活方式的改變、環境因素和生育年齡的推遲,人們對先進的生殖健康解決方案有著很高的需求。
預計到 2025 年,生育診所產業將創造 176 億美元的收入,主導服務業,其專業設施配備生殖內分泌學家、胚胎學家和生育專家,提供高成功率。
北美輔助生殖技術(ART)市場仍然是最大的區域市場,這得益於其完善的醫療保健基礎設施、較高的患者意識、健全的法規結構以及人工智慧胚胎選擇、基因篩檢和冷凍保存等創新技術的廣泛應用。不孕症盛行率的上升以及政府和保險公司為降低經濟門檻而採取的舉措,進一步推動了市場對該技術的接受度。
目錄
第1章調查方法和範圍
第2章執行摘要
第3章業界考察
- 生態系分析
- 產業影響因素
- 促進要素
- 人口不孕症日益增多
- 人口結構變化對輔助生殖技術(ART)接受度的影響
- 輔助生殖技術的進步
- 人們對輔助生殖技術的認知不斷提高
- 產業潛在風險與挑戰
- 嚴格的法規環境
- 輔助生殖技術高成本
- 市場機遇
- 個人化和精準生殖醫學
- 促進要素
- 成長潛力分析
- 監管環境
- 北美洲
- 歐洲
- 亞太地區
- 技術格局
- 未來市場趨勢
- 波特五力分析
- PESTEL 分析
第4章 競爭情勢
- 介紹
- 公司市佔率分析
- 世界
- 北美洲
- 歐洲
- 企業矩陣分析
- 主要市場公司的競爭分析
- 競爭定位矩陣
- 重大進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章 2021-2034年按產品分類的市場估算與預測
- 培養基
- 胚胎培養基
- 冷凍保存介質
- 精液處理培養基
- 卵子處理培養基
- 裝置
- 玻璃化設備
- 培養箱
- 精子分析系統
- 微型操縱器
- 其他設備
- 消耗品
- 導管
- 針
- 儲存容器
- 其他消耗品
- 添加劑
第6章 依治療方法分類的市場估計與預測,2021-2034年
- 科技
- 體外受精(IVF)
- 體外受精(IVF)結合卵胞漿內單一精子注射(ICSI)
- 無需ICSI的試管嬰兒
- 人工授精 - 陰道內授精 (AI-IUI)
- 體外受精(IVF)
- 按類型
- 新鮮精子捐贈者
- 新鮮非提供者
- 冷凍捐贈者
- 冷凍非捐贈者
7. 依最終用途分類的市場估計與預測,2021-2034 年
- 醫院
- 不孕不育診所
- 冷凍庫
- 其他用途
第8章 2021-2034年各地區市場估算與預測
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- Esco Micro
- Gynotec
- Hamilton Thorne
- Indira IVF Hospital
- Ivy Fertility
- Kitazato
- Laboratoire
- Liverpool Partners Group
- Merck
- MISTRAL FERTILITY CLINICS
- NidaCon International
- 庫柏公司
- Thermo Fisher Scientific
- Vitrolife Group
- Western Fertility Institute
The Global Assisted Reproductive Technology (ART) Market was valued at USD 38.9 billion in 2025 and is estimated to grow at a CAGR of 7.6% to reach USD 79.8 billion by 2035.

The market expansion is fueled by continuous technological advancements that enhance treatment effectiveness and patient experience. Innovations such as AI-driven embryo selection, time-lapse imaging, and preimplantation genetic testing (PGT) are improving implantation outcomes while reducing cycle failures, making ART increasingly reliable and appealing. Assisted reproductive technology encompasses medical procedures designed to help individuals or couples conceive when natural conception is challenging, involving the manipulation of eggs, sperm, or embryos outside the body. Changing social norms and greater acceptance of diverse family structures, including single-parent and same-sex families, have broadened the demand for ART solutions. Rising awareness, evolving parental expectations, and growing acceptance of reproductive alternatives have collectively accelerated market growth, creating opportunities for clinics, technology providers, and ancillary service providers globally.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $38.9 Billion |
| Forecast Value | $79.8 Billion |
| CAGR | 7.6% |
The technology segment reached USD 31.2 billion in 2025, reflecting high demand for advanced reproductive solutions driven by lifestyle changes, environmental factors, and delayed parenthood.
The fertility clinics segment generated USD 17.6 billion in 2025, dominating the service landscape as specialized centers employing reproductive endocrinologists, embryologists, and fertility experts to deliver high success rates.
North America Assisted Reproductive Technology (ART) Market remains the largest regional market, supported by a robust healthcare infrastructure, high patient awareness, strong regulatory frameworks, and widespread adoption of innovative technologies such as AI-based embryo selection, genetic screening, and cryopreservation techniques. Rising infertility prevalence and government or insurance initiatives to reduce financial barriers further strengthen market adoption.
Prominent players in the Global Assisted Reproductive Technology (ART) Market include Hamilton Thorne, Western Fertility Institute, Kitazato, Indira IVF Hospital, Gynotec, Merck, Esco Micro, Ivy Fertility, Laboratoire, Liverpool Partners Group, Thermo Fisher Scientific, The Cooper Companies, Vitrolife Group, NidaCon International, and Mistral Fertility Clinics. Companies in the Global Assisted Reproductive Technology (ART) Market are reinforcing their position through investment in research and development to enhance treatment success rates and patient outcomes. Strategic partnerships with fertility clinics, hospitals, and diagnostic labs help expand service networks and improve accessibility. Adoption of AI, genetic testing, and cryopreservation innovations enables differentiation through advanced and efficient treatment solutions. Firms are also focusing on geographic expansion into high-demand regions and providing patient education programs to build trust and awareness.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product trends
- 2.2.3 Procedures trends
- 2.2.4 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of infertility across population base
- 3.2.1.2 Demographical shift towards acceptance of assisted reproductive technology (ART)
- 3.2.1.3 Technological advancements in assisted reproductive technology
- 3.2.1.4 Rising awareness regarding assisted reproductive technology
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory scenario
- 3.2.2.2 High cost of ART procedures
- 3.2.3 Market opportunity
- 3.2.3.1 Personalized and precision reproductive medicine
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Media
- 5.2.1 Embryo culture media
- 5.2.2 Cryopreservation media
- 5.2.3 Semen processing media
- 5.2.4 Ovum processing media
- 5.3 Instrument
- 5.3.1 Vitrification devices
- 5.3.2 Incubators
- 5.3.3 Sperm analyzer system
- 5.3.4 Micromanipulators
- 5.3.5 Other instruments
- 5.4 Consumables
- 5.4.1 Catheters
- 5.4.2 Needles
- 5.4.3 Storage containers
- 5.4.4 Other consumables
- 5.5 Supplements
Chapter 6 Market Estimates and Forecast, By Procedure, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Technology
- 6.2.1 In-vitro fertilization (IVF)
- 6.2.1.1 IVF with intracytoplasmic sperm injection (ICSI)
- 6.2.1.2 IVF without ICSI
- 6.2.2 Artificial insemination - intrauterine insemination (AI-IUI)
- 6.2.1 In-vitro fertilization (IVF)
- 6.3 Type
- 6.3.1 Fresh donor
- 6.3.2 Fresh non donor
- 6.3.3 Frozen donor
- 6.3.4 Frozen non donor
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Fertility clinics
- 7.4 Cryobanks
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Esco Micro
- 9.2 Gynotec
- 9.3 Hamilton Thorne
- 9.4 Indira IVF Hospital
- 9.5 Ivy Fertility
- 9.6 Kitazato
- 9.7 Laboratoire
- 9.8 Liverpool Partners Group
- 9.9 Merck
- 9.10 MISTRAL FERTILITY CLINICS
- 9.11 NidaCon International
- 9.12 The Cooper Companies
- 9.13 Thermo Fisher Scientific
- 9.14 Vitrolife Group
- 9.15 Western Fertility Institute












