|
市場調查報告書
商品編碼
1892753
外分泌性胰臟功能不全治療市場機會、成長促進因素、產業趨勢分析及預測(2026-2035年)Exocrine Pancreatic Insufficiency Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035 |
||||||
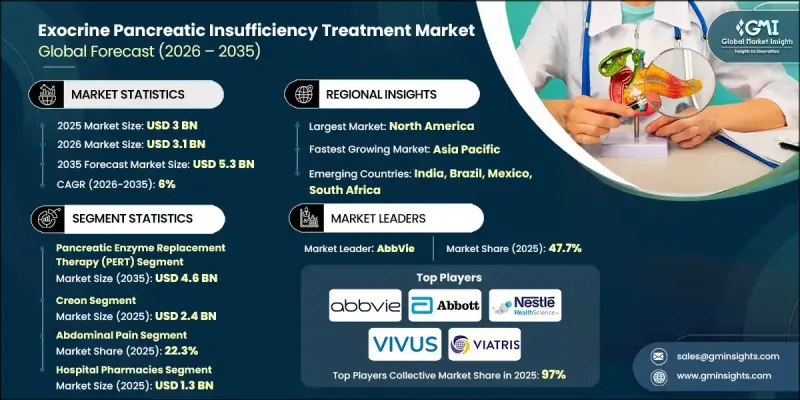
2025 年全球外分泌胰臟功能不全治療市場價值為 30 億美元,預計到 2035 年將以 6% 的複合年成長率成長至 53 億美元。
慢性胰臟炎、囊性纖維化和人口老化的日益普遍,以及診斷技術的進步和藥物創新,共同推動了市場成長。診斷方法的改進使得外分泌胰臟功能不全(EPI)的早期發現成為可能,從而改善治療效果並提高患者的生活品質。研究日益關注尋找精準的生物標記物,以準確評估胰臟功能。糞便彈性蛋白酶-1水平低是胰臟功能不全的可靠指標,為侵入性檢查提供了更簡單、更適合門診的替代方案。 EPI的治療包括藥物和飲食管理,以補充營養消化吸收所必需的胰酶。及時介入有助於減輕症狀、改善營養吸收並顯著提升患者的整體健康狀況。人們對胰臟疾病的認知不斷提高,以及對有效治療方案的需求日益成長,進一步推動了全球對這些療法的需求。
| 市場範圍 | |
|---|---|
| 起始年份 | 2025 |
| 預測年份 | 2026-2035 |
| 起始值 | 30億美元 |
| 預測值 | 53億美元 |
| 複合年成長率 | 6% |
2025年,胰酶替代療法(PERT)市場規模為26億美元,預計2035年將達46億美元。外分泌胰臟功能不全(EPI)是指胰臟無法產生或輸送足夠的消化酶,導致消化不良和吸收不良。常見的潛在原因包括慢性胰臟炎、胰臟癌、囊性纖維化和糖尿病。 PERT透過口服胰脂肪酶(一種脂肪酶、蛋白酶和澱粉酶的混合物)來幫助患者恢復正常的消化功能。
預計到2025年,腹痛類藥物市佔率將達到22.3%。腹痛是外分泌胰臟功能不全(EPI)的常見症狀,由酵素分泌不足和消化不完全引起。胰酶替代療法(PERT)透過改善消化和減少腸道內未消化食物的積聚來緩解這種不適。準確控制酵素製劑的劑量和服用時間(與餐點同服)對於有效控制症狀至關重要。
2025年,美國外分泌性胰臟功能不全治療市場規模預計將達16.2億美元。胰臟疾病(包括胰臟癌和囊性纖維化)發生率的上升是該市場的主要成長促進因素。隨著這些疾病盛行率的持續成長,對先進且有效的外分泌性胰臟功能不全治療方案的需求也不斷擴大,進一步推動了北美市場的成長。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 產業影響因素
- 成長促進因素
- 慢性胰臟炎(CP)和囊性纖維化盛行率不斷上升
- 人口老化日益加劇
- 診斷技術的進步
- 製藥業的技術進步
- 產業陷阱與挑戰
- 治療費用高昂
- 認知和教育程度有限
- 市場機遇
- 對個人化醫療的需求日益成長
- 成長促進因素
- 成長潛力分析
- 監管環境
- 北美洲
- 歐洲
- 亞太地區
- 未來市場趨勢
- 管道分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:依治療方法分類,2022-2035年
- 營養管理
- 胰酵素替代療法(PERT)
第6章:市場估算與預測:依藥物類型分類,2022-2035年
- 克瑞翁
- Zenpep
- 胰臟
- 維奧卡斯
- 其他藥物類型
第7章:市場估算與預測:依症狀分類,2022-2035年
- 腹痛
- 便秘
- 腹瀉
- 脂肪便
- 減肥
- 其他症狀
第8章:市場估算與預測:依配銷通路分類,2022-2035年
- 醫院藥房
- 零售藥局
- 網路藥局
第9章:市場估計與預測:依地區分類,2022-2035年
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- Abbott Laboratories
- AbbVie
- Digestive Care
- Essential Pharma
- Eisai
- Nestle
- Nordmark Pharma
- Sun Pharmaceutical Industries
- Viatris
- VIVUS
- Zentiva Pharma
The Global Exocrine Pancreatic Insufficiency Treatment Market was valued at USD 3 billion in 2025 and is estimated to grow at a CAGR of 6% to reach USD 5.3 billion by 2035.

Market growth is fueled by the rising prevalence of chronic pancreatitis, cystic fibrosis, and an aging population, combined with advancements in diagnostic technologies and pharmaceutical innovations. Enhanced diagnostic methods allow earlier detection of EPI, improving treatment outcomes and patient quality of life. Research increasingly focuses on identifying precise biomarkers to assess pancreatic function accurately. Low fecal elastase-1 levels serve as a reliable indicator of pancreatic insufficiency, offering a simpler, outpatient-friendly alternative to invasive procedures. EPI treatment involves medical and dietary management to replace deficient pancreatic enzymes essential for nutrient digestion and absorption. Timely intervention helps reduce symptoms, improve nutrient uptake, and significantly enhance overall patient well-being. The growing awareness of pancreatic disorders and the need for effective treatment solutions is further driving global demand for these therapies.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $3 Billion |
| Forecast Value | $5.3 Billion |
| CAGR | 6% |
The pancreatic enzyme replacement therapy (PERT) segment accounted for USD 2.6 billion in 2025 and is projected to reach USD 4.6 billion by 2035. EPI occurs when the pancreas fails to produce or deliver sufficient digestive enzymes, leading to maldigestion and malabsorption. Common underlying conditions include chronic pancreatitis, pancreatic cancer, cystic fibrosis, and diabetes. PERT provides patients with oral administration of pancrelipase, a combination of lipase, protease, and amylase, to restore normal digestive function.
The abdominal pain segment held a 22.3% share in 2025. Abdominal pain is a frequent symptom of EPI, arising from insufficient enzyme production and incomplete digestion. PERT alleviates this discomfort by improving digestion and reducing undigested food accumulation in the intestines. Accurate dosing and timing of enzyme intake with meals are essential for effective symptom control.
U.S. Exocrine Pancreatic Insufficiency Treatment Market was valued at USD 1.62 billion in 2025. Rising incidences of pancreatic disorders, including pancreatic cancer and cystic fibrosis, are primary growth drivers. As the prevalence of these conditions continues to increase, the demand for advanced and effective EPI therapies is expanding, supporting further market growth in North America.
Prominent companies active in the Global Exocrine Pancreatic Insufficiency Treatment Market include Sun Pharmaceutical Industries, Zentiva Pharma, Nordmark Pharma, Abbott Laboratories, Viatris, Digestive Care, Essential Pharma, Eisai, AbbVie, VIVUS, and Nestle. Companies in the EPI treatment sector are enhancing their market presence through targeted product innovation and portfolio expansion. Many are investing in next-generation enzyme formulations and combination therapies to improve efficacy and patient adherence. Research and development initiatives focus on identifying biomarkers and improving diagnostic tools, enabling earlier and more precise treatment. Strategic partnerships with healthcare providers and collaborations with research institutions strengthen credibility and market access. Manufacturers are also leveraging digital platforms and telemedicine solutions to educate patients and support treatment compliance. Expanding geographic presence in high-prevalence regions, optimizing supply chains, and aligning with regulatory standards ensures broader reach and trust.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Treatment trends
- 2.2.3 Drug type trends
- 2.2.4 Symptom trends
- 2.2.5 Distribution channel trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of chronic pancreatitis (CP) and cystic fibrosis
- 3.2.1.2 Growing aging population
- 3.2.1.3 Advancements in diagnosis
- 3.2.1.4 Technological advancements in pharmaceutical industry
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of treatment
- 3.2.2.2 Limited awareness and education
- 3.2.3 Market opportunities
- 3.2.3.1 Growing demand for personalized medicine
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Future market trends
- 3.6 Pipeline analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2025
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Treatment, 2022 - 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Nutritional management
- 5.3 Pancreatic enzyme replacement therapy (PERT)
Chapter 6 Market Estimates and Forecast, By Drug Type, 2022 - 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Creon
- 6.3 Zenpep
- 6.4 Pancreaze
- 6.5 Viokace
- 6.6 Other drug types
Chapter 7 Market Estimates and Forecast, By Symptom, 2022 - 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Abdominal pain
- 7.3 Constipation
- 7.4 Diarrhea
- 7.5 Fatty stools
- 7.6 Weight loss
- 7.7 Other symptoms
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2022 - 2035 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2022 - 2035 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 AbbVie
- 10.3 Digestive Care
- 10.4 Essential Pharma
- 10.5 Eisai
- 10.6 Nestle
- 10.7 Nordmark Pharma
- 10.8 Sun Pharmaceutical Industries
- 10.9 Viatris
- 10.10 VIVUS
- 10.11 Zentiva Pharma