 |
市場調查報告書
商品編碼
1892750
體外膜氧合市場機會、成長促進因素、產業趨勢分析及預測(2026-2035年)Extracorporeal Membrane Oxygenation Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035 |
||||||
2025 年全球體外膜氧合市場價值為 4.755 億美元,預計到 2035 年將以 4.5% 的複合年成長率成長至 7.216 億美元。
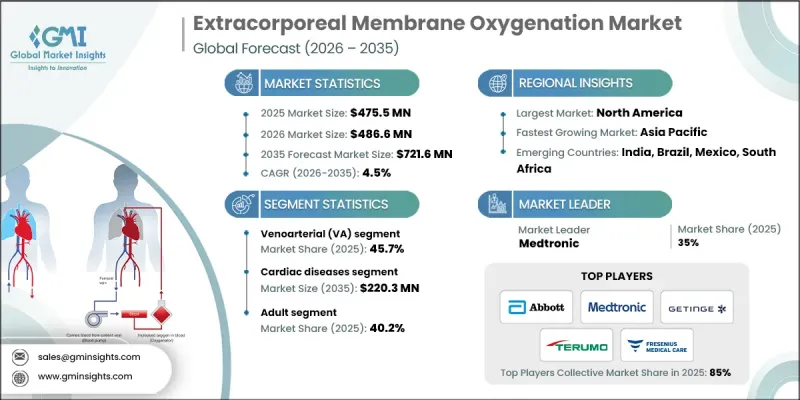
市場成長的促進因素包括心肺疾病和呼吸衰竭的日益普遍、體外膜氧合(ECMO)系統技術的快速發展,以及患者對這些維生設備益處的認知不斷提高。 ECMO系統透過人工膜將血液循環至體外,暫時取代心臟和肺部的功能,補充氧氣並清除二氧化碳。當機械通氣等傳統療法不足以維持生命時,這些設備在重症監護中至關重要,可為患者康復或等待器官移植提供支持。全球醫療素養的提高和政府措施正在推動ECMO的普及應用,而先進治療方案的可及性不斷提高,也確保了ECMO被公認為治療嚴重心肺衰竭的重要手段。
| 市場範圍 | |
|---|---|
| 起始年份 | 2025 |
| 預測年份 | 2026-2035 |
| 起始值 | 4.755億美元 |
| 預測值 | 7.216億美元 |
| 複合年成長率 | 4.5% |
2025年,靜脈-動脈(VA)體外膜氧合(ECMO)市佔率佔比達45.7%。 VA ECMO為重度心臟衰竭患者(常伴隨呼吸功能障礙)提供心肺支持,廣泛用於治療心因性休克、心臟停止等疾病,或作為心臟移植的過渡治療。其日益普及得益於其在危重心臟疾病(包括術後併發症和嚴重心肌功能障礙)治療中展現出的顯著療效。
預計到2025年,成人市場將佔據40.2%的佔有率,這反映出由於生活方式相關的風險因素,成人中嚴重心臟和呼吸系統疾病的盛行率更高。體外膜氧合(ECMO)作為一種首選的高級重症監護解決方案,在三級醫院的應用日益廣泛,它可在高風險手術期間提供挽救生命的支持,或作為器官移植的過渡手段。
2025年,北美體外膜氧合市場將佔據58.2%的市佔率。該地區的成長得益於先進的醫療保健基礎設施、不斷上升的心肺疾病發病率以及持續的技術創新,而心血管疾病負擔的加重將進一步加速該技術的普及應用。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 產業影響因素
- 成長促進因素
- 心肺疾病和呼吸衰竭病例增加
- ECMO設備的技術進步
- 患者對體外膜氧合(ECMO)裝置益處的認知不斷提高
- 全球政府措施和計劃日益增多
- 產業陷阱與挑戰
- 設備成本高
- 缺乏熟練專業人員
- ECMO設備相關風險
- 市場機遇
- 居家式和長期體外膜氧合(ECMO)解決方案
- 與人工智慧和數位監控的整合
- 成長促進因素
- 成長潛力分析
- 監管環境
- 技術進步
- 當前技術趨勢
- 新興技術
- 供應鏈分析
- 報銷方案
- 2025年定價分析
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估算與預測:依模式分類,2022-2035年
- 靜脈-動脈(VA)
- 靜脈-靜脈(VV)
- 動靜脈(AV)
第6章:市場估算與預測:依應用領域分類,2022-2035年
- 心臟疾病
- 呼吸系統疾病
- 體外心肺復甦術(ECPR)
第7章:市場估計與預測:依病患群體分類,2022-2035年
- 嬰兒
- 兒科
- 成人
第8章:市場估算與預測:依地區分類,2022-2035年
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第9章:公司簡介
- Abbott Laboratories
- Abiomed
- Eurosets
- Braile Biomedica
- Edwards Lifesciences Corporation
- Fresenius Medical Care AG & Co. KGaA
- Getinge AB
- Hemovent GmbH
- LivaNova PLC
- Medtronic
- MicroPort Scientific Corporation
- Nipro
- SB-Kawasumi Laboratories, Inc.
- Senko Medical Instrument Mfg. Co., Ltd.
- Spectrum Medical, Terumo Corporation
- Weigao Group Co., Ltd.
The Global Extracorporeal Membrane Oxygenation Market was valued at USD 475.5 million in 2025 and is estimated to grow at a CAGR of 4.5% to reach USD 721.6 million by 2035.

Market growth is driven by the rising prevalence of cardiopulmonary diseases and respiratory failures, rapid technological advancements in ECMO systems, and increasing awareness among patients regarding the benefits of these life-support devices. ECMO systems temporarily take over the function of the heart and lungs by circulating blood outside the body through an artificial membrane, adding oxygen and removing carbon dioxide. These devices are crucial in critical care when conventional therapies, such as mechanical ventilation, are insufficient, offering support during recovery or while awaiting organ transplantation. Enhanced healthcare literacy and government initiatives worldwide are encouraging adoption, while improved access to advanced treatment options ensures that ECMO is recognized as a vital intervention for severe cardiac and respiratory failures.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $475.5 Million |
| Forecast Value | $721.6 Million |
| CAGR | 4.5% |
The venoarterial (VA) ECMO segment accounted for a 45.7% share in 2025. VA ECMO delivers cardiopulmonary support for patients with severe cardiac failure, often combined with respiratory compromise, and is widely used for conditions such as cardiogenic shock, cardiac arrest, or as a bridge to heart transplantation. Its growing adoption is driven by proven effectiveness in critical cardiac conditions, including post-surgical complications and severe myocardial dysfunction.
The adult segment captured a 40.2% share in 2025, reflecting the higher prevalence of severe cardiac and respiratory conditions among adults due to lifestyle-related risk factors. ECMO adoption is increasing in tertiary hospitals as a preferred advanced critical care solution, providing life-saving support during high-risk surgeries or as a bridge to transplantation.
North America Extracorporeal Membrane Oxygenation Market held a 58.2% share in 2025. Growth in the region is fueled by advanced healthcare infrastructure, rising cardiopulmonary disease incidence, and continuous technological innovation, with cardiovascular disease burden further accelerating adoption.
Key players in the Global Extracorporeal Membrane Oxygenation Market include Abbott Laboratories, Getinge AB, Fresenius Medical Care AG & Co. KGaA, Medtronic, Eurosets, LivaNova PLC, Abiomed, Braile Biomedica, Hemovent GmbH, MicroPort Scientific Corporation, Nipro, Edwards Lifesciences Corporation, SB-Kawasumi Laboratories, Inc., and Senko Medical Instrument Mfg. Co., Ltd., Spectrum Medical, Terumo Corporation, and Weigao Group Co., Ltd. Companies in the Extracorporeal Membrane Oxygenation Market strengthen their presence by investing heavily in R&D to develop innovative, energy-efficient, and compact ECMO systems with improved safety features. Expanding global distribution networks ensures timely delivery and service support for hospitals and critical care centers. Strategic partnerships with healthcare providers and government agencies enable long-term adoption contracts and market penetration. Manufacturers focus on enhancing product usability, reducing complications, and providing training programs for medical staff to improve device acceptance.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Modality trends
- 2.2.3 Application trends
- 2.2.4 Patient population trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increase in cases of cardiopulmonary diseases and respiratory failures
- 3.2.1.2 Technological advancements in ECMO devices
- 3.2.1.3 Rising patient awareness about benefits delivered by extracorporeal membrane oxygenation devices
- 3.2.1.4 Rising government initiatives and programs worldwide
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of equipment
- 3.2.2.2 Dearth of skilled professionals
- 3.2.2.3 Risks related to ECMO device
- 3.2.3 Market opportunities
- 3.2.3.1 Home-based and long-term ECMO solutions
- 3.2.3.2 Integration with artificial intelligence and digital monitoring
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Reimbursement scenario
- 3.8 Pricing analysis, 2025
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2025
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Modality, 2022 - 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Venoarterial (VA)
- 5.3 Veno-venous (VV)
- 5.4 Arteriovenous (AV)
Chapter 6 Market Estimates and Forecast, By Application, 2022 - 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Cardiac diseases
- 6.3 Respiratory diseases
- 6.4 Extracorporeal cardiopulmonary resuscitation (ECPR)
Chapter 7 Market Estimates and Forecast, By Patient Population, 2022 - 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Infant
- 7.3 Pediatric
- 7.4 Adult
Chapter 8 Market Estimates and Forecast, By Region, 2022 - 2035 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 Saudi Arabia
- 8.6.2 South Africa
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott Laboratories
- 9.2 Abiomed
- 9.3 Eurosets
- 9.4 Braile Biomedica
- 9.5 Edwards Lifesciences Corporation
- 9.6 Fresenius Medical Care AG & Co. KGaA
- 9.7 Getinge AB
- 9.8 Hemovent GmbH
- 9.9 LivaNova PLC
- 9.10 Medtronic
- 9.11 MicroPort Scientific Corporation
- 9.12 Nipro
- 9.13 SB-Kawasumi Laboratories, Inc.
- 9.14 Senko Medical Instrument Mfg. Co., Ltd.
- 9.15 Spectrum Medical, Terumo Corporation
- 9.16 Weigao Group Co., Ltd.







