 |
市場調查報告書
商品編碼
1885919
耳鼻喉科器材市場機會、成長促進因素、產業趨勢分析及預測(2025-2034年)ENT Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
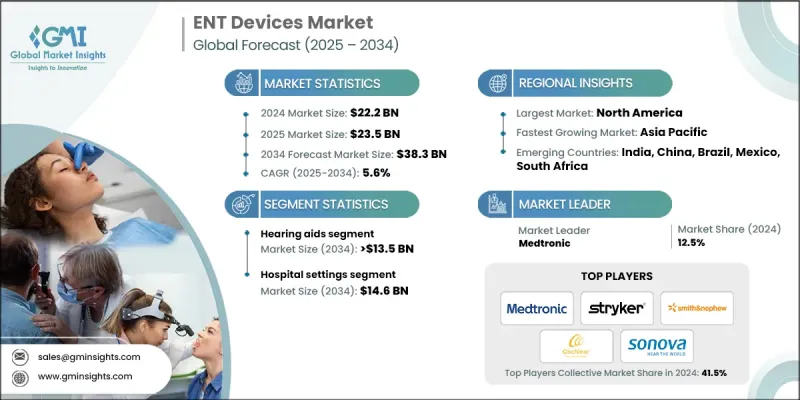
2024 年全球耳鼻喉科設備市場價值為 222 億美元,預計到 2034 年將以 5.6% 的複合年成長率成長至 383 億美元。
耳鼻喉疾病盛行率上升、老年人口不斷成長以及對微創手術的需求日益增加,是推動市場成長的主要因素。該市場為醫療服務提供者、生命科學公司、支付方和技術公司提供創新解決方案,以改善患者護理、提高合規性並提升營運效率。主要產品包括內視鏡系統、人工耳蝸、微創手術器械、診斷設備和數位化耳鼻喉平台,旨在提高手術精準度、疾病管理水平和整體生活品質。全球人口老化導致聽力損失、慢性鼻竇炎和平衡障礙的發生率上升,增加了對耳鼻喉科干預的需求。都市化和生活方式的改變也導致呼吸系統和過敏性疾病的發生率上升,擴大了患者群體。由於恢復時間短、併發症風險低、療效好,臨床醫生和患者越來越傾向於選擇微創技術,這推動了對專用耳鼻喉科設備的需求。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 222億美元 |
| 預測值 | 383億美元 |
| 複合年成長率 | 5.6% |
2024年,助聽器市佔率達37.5%。老年人聽力損失盛行率的上升以及包括數位化和人工智慧助聽器在內的技術進步推動了這一成長。此細分市場涵蓋耳背式(BTE)、耳內式/耳道式(RITE/RIC)、全耳道式/隱形耳道式(CIC/IIC)、耳內式(ITE)和耳道式(ITC)助聽器。人們對聽力健康的日益重視以及對先進和傳統助聽器的需求,正在推動醫院、診所和家庭護理機構對助聽器的廣泛應用。
2024年,耳鼻喉科診斷設備市場規模達到58億美元,預計2025年至2034年將以6.2%的複合年成長率成長。該市場涵蓋硬式和軟式內視鏡、聽力篩檢設備以及機器人輔助內視鏡。對耳鼻喉科疾病早期檢測和精準診斷需求的不斷成長,是推動該市場成長的主要動力。
2024年,北美耳鼻喉科器械市佔率達到38.6%。其市場主導地位得益於完善的醫療保健基礎設施、先進醫療技術的應用以及高昂的醫療支出。慢性鼻竇炎、聽力損失和睡眠呼吸中止症等耳鼻喉科疾病病例的增加,推動了對精密診斷和治療設備的需求。包括高清內視鏡、機器人手術系統和人工智慧診斷工具在內的技術創新,提高了手術的準確性和效率,促進了這些設備在醫院、門診手術中心和診所的應用。
耳鼻喉科器材市場的主要參與者包括美敦力(Medtronic)、德索特醫療(DeSoutter Medical)、捷邁邦美(Zimmer Biomet)、波士頓科學(Boston Scientific)、史賽克(Stryker)、強生(Ethicon)、韋爾奇艾林(Welch Allyn,Hillromance)、索諾瓦(S. Medical、奧林巴斯(Olympus)、史密斯醫療(Smith & Nephew)、Nouvag、Vega Medical 和 WestCMR。這些公司正採取多種策略來鞏固自身地位並擴大市場佔有率。他們大力投資研發,以推出創新且技術先進的產品。策略合作、協作和收購有助於拓展地域覆蓋範圍和分銷網路。此外,各公司也致力於透過數位化、人工智慧和微創解決方案來擴展產品組合,以滿足不同患者的需求。重視監管合規、品質認證和永續發展措施有助於提升公司信譽。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 產業影響因素
- 成長促進因素
- 全球耳鼻喉疾病盛行率不斷上升
- 老年人口不斷增加
- 耳鼻喉科設備的技術進步
- 對微創耳鼻喉科手術的需求不斷成長
- 產業陷阱與挑戰
- 高昂的手術和器械成本
- 全球範圍內的社會歧視
- 市場機遇
- 人工智慧診斷的整合
- 遠距耳鼻喉科會診量不斷上升
- 成長促進因素
- 成長潛力分析
- 監管環境
- 北美洲
- 歐洲
- 亞太地區
- 拉丁美洲
- 技術格局
- 當前技術趨勢
- 微創耳鼻喉科手術器械和內視鏡系統的發展
- 實現遠距耳鼻喉科會診和遠距診斷的數位化耳鼻喉科平台
- 對患者友善的聽力植入體和攜帶式聽力設備
- 新興技術
- 人工智慧驅動的耳鼻喉科診斷和預測性疾病管理
- 穿戴式及連網式聽力及平衡設備
- 具有自適應治療和個人化治療模式的智慧耳鼻喉科設備
- 當前技術趨勢
- 差距分析
- 波特的分析
- PESTEL 分析
- 未來市場趨勢
- 人工智慧整合式耳鼻喉科設備在疾病早期檢測和個人化治療方案製定方面的應用日益廣泛
- 遠端耳鼻喉科平台和遠端監測解決方案在以患者為中心的護理中得到更廣泛的應用
- 微創、智慧、穿戴式耳鼻喉科設備的普及提高了手術效率和患者依從性。
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 公司市佔率分析
- 全球的
- 北美洲
- 歐洲
- 亞太地區
- 拉丁美洲
- 競爭定位矩陣
- 主要市場參與者的競爭分析
- 關鍵進展
- 併購
- 合作夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估算與預測:依設備類型分類,2021-2034年
- 主要趨勢
- 助聽器
- 耳後式(BTE)
- 耳內式/耳道式受話器(RITE/RIC)
- 完全管內式/隱形管內式 (CIC/IIC)
- 耳內式(ITE)
- 運河內(ITC)
- 耳鼻喉科診斷設備
- 硬式內視鏡
- 喉鏡
- 鼻鏡
- 耳科內視鏡
- 軟性內視鏡
- 聽力篩檢設備
- 機器人輔助內視鏡
- 硬式內視鏡
- 耳鼻喉外科器械
- 鼻竇擴張裝置
- 電動手術器械
- 耳科鑽頭
- 射頻手柄
- 耳鼻喉科手用器械
- 鼓膜置管
- 鼻腔填塞裝置
- 聽力植入
- 人工耳蝸
- 骨錨式助聽系統
- 聽覺腦幹植入
- 中耳植入物
- 語音假體裝置
- 鼻夾板
- 外鼻夾板
- 鼻內夾板
第6章:市場估算與預測:依最終用途分類,2021-2034年
- 主要趨勢
- 醫院
- 門診手術中心
- 耳鼻喉科診所
- 居家照護
第7章:市場估計與預測:依地區分類,2021-2034年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第8章:公司簡介
- Atos Medical
- Boston Scientific
- Cochlear
- DeSoutter Medical
- Johnson & Johnson (Ethicon)
- Lumenis
- Medtronic
- Meril Life Sciences
- Narang Medical
- Nouvag
- Olympus
- Smith & Nephew
- Sonova
- Stryker
- Vega Medical
- Welch Allyn (Hillrom)
- WestCMR
- Zimmer Biomet
The Global ENT Devices Market was valued at USD 22.2 billion in 2024 and is estimated to grow at a CAGR of 5.6% to reach USD 38.3 billion by 2034.

Growth is driven by the rising prevalence of ENT disorders, an expanding geriatric population, and increasing demand for minimally invasive procedures. The market offers innovative solutions to healthcare providers, life science companies, payers, and technology firms to improve patient care, regulatory adherence, and operational efficiency. Key offerings include endoscopy systems, hearing implants, minimally invasive surgical tools, diagnostic devices, and digital ENT platforms designed to enhance procedural precision, disease management, and overall quality of life. The global aging population is contributing to higher incidences of hearing loss, chronic sinusitis, and balance disorders, increasing the need for ENT interventions. Urbanization and lifestyle changes are also leading to higher rates of respiratory and allergic conditions, expanding the patient base. Clinicians and patients increasingly prefer minimally invasive techniques due to shorter recovery times, lower complication risks, and improved outcomes, driving the demand for specialized ENT devices.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $22.2 Billion |
| Forecast Value | $38.3 Billion |
| CAGR | 5.6% |
The hearing aids segment held a 37.5% share in 2024. Growth is fueled by the rising prevalence of hearing loss among the elderly and technological advancements, including digital and AI-enabled hearing aids. This segment includes behind-the-ear (BTE), receiver-in-ear/receiver-in-canal (RITE/RIC), completely-in-canal/invisible-in-canal (CIC/IIC), in-the-ear (ITE), and in-the-canal (ITC) devices. Increasing awareness of hearing health and the demand for advanced and conventional hearing devices are driving adoption across hospitals, clinics, and home care settings.
The diagnostic ENT devices segment generated USD 5.8 billion in 2024 and is projected to grow at a CAGR of 6.2% from 2025 to 2034. It includes rigid and flexible endoscopes, hearing screening devices, and robot-assisted endoscopes. Rising demand for early detection and accurate diagnosis of ENT disorders is fueling growth in this segment.
North America ENT Devices Market held 38.6% share in 2024. Market dominance is supported by a well-established healthcare infrastructure, adoption of advanced medical technologies, and high healthcare expenditure. Increasing cases of ENT disorders such as chronic sinusitis, hearing loss, and sleep apnea are boosting demand for sophisticated diagnostic and treatment devices. Technological innovations, including high-definition endoscopes, robotic surgical systems, and AI-powered diagnostic tools, improve procedural accuracy and efficiency, driving adoption in hospitals, ambulatory surgical centers, and clinics.
Key players in the ENT Devices Market include Medtronic, DeSoutter Medical, Zimmer Biomet, Boston Scientific, Stryker, Johnson & Johnson (Ethicon), Welch Allyn (Hillrom), Sonova, Lumenis, Atos Medical, Narang Medical, Olympus, Smith & Nephew, Nouvag, Vega Medical, and WestCMR. Companies in the ENT devices market are employing multiple strategies to strengthen their position and expand their footprint. They are investing heavily in research and development to introduce innovative and technologically advanced products. Strategic partnerships, collaborations, and acquisitions help broaden geographic presence and distribution networks. Companies are also focusing on expanding product portfolios with digital, AI-enabled, and minimally invasive solutions to meet diverse patient needs. Emphasis on regulatory compliance, quality certifications, and sustainability initiatives enhances credibility.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product type trends
- 2.2.3 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of ENT disorders globally
- 3.2.1.2 Increasing geriatric population
- 3.2.1.3 Technological advancements in the ENT devices
- 3.2.1.4 Rising demand for minimally invasive ENT procedures
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High procedure and instruments cost
- 3.2.2.2 Social stigma across the globe
- 3.2.3 Market opportunities
- 3.2.3.1 Integration of AI diagnostics
- 3.2.3.2 Rising tele-ENT consultations
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 LAMEA
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.1.1 Growth of minimally invasive ENT surgical instruments and endoscopic systems
- 3.5.1.2 Digital ENT platforms enabling tele-ENT consultations and remote diagnostics
- 3.5.1.3 Patient-friendly hearing implants and portable audiology devices
- 3.5.2 Emerging technologies
- 3.5.2.1 AI-powered ENT diagnostics and predictive disease management
- 3.5.2.2 Wearable and connected hearing and balance devices
- 3.5.2.3 Smart ENT devices with adaptive treatment and personalized therapy modes
- 3.5.1 Current technological trends
- 3.6 Gap analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
- 3.9 Future market trends
- 3.9.1 Expansion of AI-integrated ENT devices for early disease detection and personalized treatment planning
- 3.9.2 Increased adoption of tele-ENT platforms and remote monitoring solutions for patient-centric care
- 3.9.3 Growth of minimally invasive, smart, and wearable ENT devices enhancing procedural efficiency and patient compliance
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.3.5 LAMEA
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launch
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Device Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Hearing aids
- 5.2.1 Behind-the-ear (BTE)
- 5.2.2 Receiver in the ear/receiver in canal (RITE/RIC)
- 5.2.3 Completely-in-the-canal/Invisible-in-canal (CIC/IIC)
- 5.2.4 In-the-ear (ITE)
- 5.2.5 In-the-canal (ITC)
- 5.3 Diagnostic ear, nose, and throat (ENT) devices
- 5.3.1 Rigid endoscopes
- 5.3.1.1 Laryngoscopes
- 5.3.1.2 Rhinoscopes
- 5.3.1.3 Otological endoscopes
- 5.3.2 Flexible endoscopes
- 5.3.3 Hearing screening devices
- 5.3.4 Robot assisted endoscopes
- 5.3.1 Rigid endoscopes
- 5.4 Surgical ear, nose, and throat (ENT) devices
- 5.4.1 Sinus dilation devices
- 5.4.2 Powered surgical instrument
- 5.4.3 Otological drill burrs
- 5.4.4 Radiofrequency handpieces
- 5.4.5 ENT hand instruments
- 5.4.6 Tympanostomy tubes
- 5.4.7 Nasal packing devices
- 5.5 Hearing implants
- 5.5.1 Cochlear implants
- 5.5.2 Bone anchored hearing system
- 5.5.3 Auditory brainstem implants
- 5.5.4 Middle ear implants
- 5.6 Voice prosthesis devices
- 5.7 Nasal splints
- 5.7.1 External nasal splints
- 5.7.2 Internal nasal splints
Chapter 6 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Hospitals
- 6.3 Ambulatory surgical centers
- 6.4 ENT clinics
- 6.5 Homecare
Chapter 7 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 North America
- 7.2.1 U.S.
- 7.2.2 Canada
- 7.3 Europe
- 7.3.1 Germany
- 7.3.2 UK
- 7.3.3 France
- 7.3.4 Spain
- 7.3.5 Italy
- 7.3.6 Netherlands
- 7.4 Asia Pacific
- 7.4.1 China
- 7.4.2 Japan
- 7.4.3 India
- 7.4.4 Australia
- 7.4.5 South Korea
- 7.5 Latin America
- 7.5.1 Brazil
- 7.5.2 Mexico
- 7.5.3 Argentina
- 7.6 Middle East and Africa
- 7.6.1 South Africa
- 7.6.2 Saudi Arabia
- 7.6.3 UAE
Chapter 8 Company Profiles
- 8.1 Atos Medical
- 8.2 Boston Scientific
- 8.3 Cochlear
- 8.4 DeSoutter Medical
- 8.5 Johnson & Johnson (Ethicon)
- 8.6 Lumenis
- 8.7 Medtronic
- 8.8 Meril Life Sciences
- 8.9 Narang Medical
- 8.10 Nouvag
- 8.11 Olympus
- 8.12 Smith & Nephew
- 8.13 Sonova
- 8.14 Stryker
- 8.15 Vega Medical
- 8.16 Welch Allyn (Hillrom)
- 8.17 WestCMR
- 8.18 Zimmer Biomet









