 |
市場調查報告書
商品編碼
1885914
醫療保健監理事務外包市場機會、成長促進因素、產業趨勢分析及預測(2025-2034年)Healthcare Regulatory Affairs Outsourcing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球醫療保健監管事務外包市場價值為 75 億美元,預計到 2034 年將以 7.6% 的複合年成長率成長至 157 億美元。
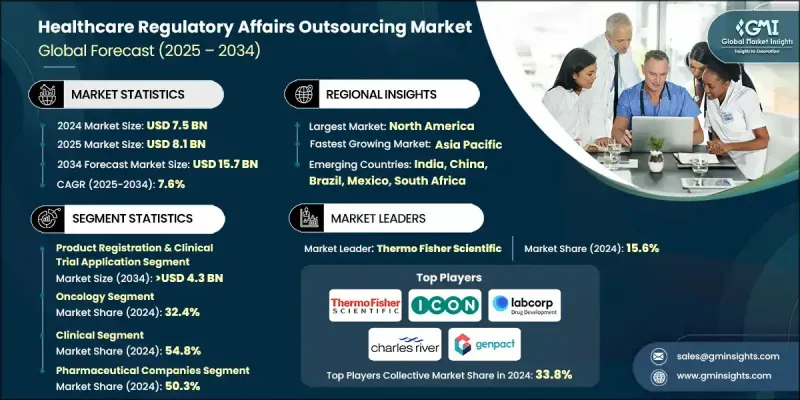
全球監管架構日益複雜,迫使製藥、生技和醫療器材公司將合規、文件編制和申報工作外包給專業合作夥伴。市場提供端到端解決方案,協助簡化監管流程,確保符合國際標準,並加速產品核准。服務包括監管策略制定、文件準備、eCTD發布、標籤管理、生命週期維護和監管情報,所有這些服務都旨在提高效率、安全性和患者療效。數位化和雲端監管資訊管理 (RIM) 系統的快速普及,以及人工智慧驅動的分析技術,正在推動對擅長管理電子申報和複雜監管工作流程的外包服務提供者的需求。此外,全球臨床試驗數量的不斷成長,包括適應性研究和分散式研究,也加重了監管負擔,促使企業依賴經驗豐富的外部合作夥伴來製定申報計劃、追蹤合規情況以及與衛生監管機構進行溝通。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 75億美元 |
| 預測值 | 157億美元 |
| 複合年成長率 | 7.6% |
2024年,產品註冊及臨床試驗申請業務佔市場佔有率的28.4%。該業務的成長主要得益於全球藥物研發活動的增加和監管要求的日益嚴格。外包對於應對複雜的申報流程至關重要,能夠確保符合FDA、EMA和PMDA等監管機構的要求,這些機構不斷收緊監管指南,以保障藥物的安全性和有效性。
2024年,腫瘤領域佔據32.4%的市場佔有率,預計在2025年至2034年間將達到54億美元。全球癌症發生率的上升、公眾意識的提高以及早期檢測計畫的發展,正在推動對創新腫瘤療法的需求。各公司正大力投資腫瘤藥物研發,並因此產生複雜的多區域監管申報。腫瘤臨床試驗的高成本和長週期,進一步促使企業將相關工作外包給在腫瘤監管方面擁有專業知識的公司。
2024年,北美醫療保健監管事務外包市佔率達46.5%。該地區的領先地位源於其強大的製藥、生物技術和醫療器材產業,這些產業需要廣泛的監管支援才能符合嚴格的監管框架。美國FDA和加拿大衛生部等機構不斷更新的法規,包括臨床試驗、生物製劑和上市後監測的指南,都增加了企業對外包夥伴的依賴。
全球醫療保健監管事務外包市場的主要參與者包括賽默飛世爾科技 (Thermo Fisher Scientific)、Clinilabs、Genpact、ICON、Syneos Health、Accell Clinical Research、Charles River Laboratories、Labcorp、NAMSA、PAREXEL、PharmaLex、ProPharma 和 Proventa。這些公司透過投資專業知識,尤其是在腫瘤和生物製劑等複雜治療領域,來增強自身實力。他們開發用於電子申報、人工智慧驅動的監管情報和基於雲端的監管資訊管理 (RIM) 系統的整合數位平台,以簡化合規工作流程。與全球製藥和生物技術公司的策略合作有助於拓展服務組合和地理覆蓋範圍。這些公司專注於監管諮詢、文件管理和生命週期維護,以增強客戶忠誠度。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 產業影響因素
- 成長促進因素
- 日益成長的遵守監管要求的需求
- 對突破性藥物和器械加快核准流程的需求激增
- 臨床試驗數量不斷增加
- 監理複雜性日益增加
- 產業陷阱與挑戰
- 資料安全和隱私問題
- 缺乏標準化
- 市場機遇
- 新興市場監管外包的擴展
- 對端對端RIM的需求不斷成長
- 成長促進因素
- 成長潛力分析
- 監管環境
- 北美洲
- 歐洲
- 亞太地區
- 拉丁美洲
- 技術格局
- 當前技術趨勢
- 採用基於雲端的監管申報和文件管理平台
- 將人工智慧和機器學習應用於監管情報和合規監控
- 整合用於全球投稿追蹤和電子投稿的數位工具
- 新興技術
- 人工智慧賦能的監管決策支援與預測性合規分析
- 利用區塊鏈技術實現安全、透明和可追溯的監管文件
- 當前技術趨勢
- 未來市場趨勢
- 監管里程碑加速市場准入
- 研發投資活動增加
- 加強監管合規性和驗證
- 投資和融資環境
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 公司市佔率分析
- 全球的
- 北美洲
- 歐洲
- 亞太地區
- 競爭定位矩陣
- 主要市場參與者的競爭分析
- 關鍵進展
- 併購
- 合作夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:依服務業分類,2021-2034年
- 主要趨勢
- 產品註冊及臨床試驗申請
- 監理諮詢/策略服務
- 提交管理
- 法律代理
- 法規撰寫與出版
- 其他服務
第6章:市場估算與預測:依指示劑分類,2021-2034年
- 主要趨勢
- 腫瘤學
- 神經病學
- 心臟病學
- 免疫學
- 其他跡象
第7章:市場估算與預測:依產品階段分類,2021-2034年
- 主要趨勢
- 臨床前
- 臨床
- 上市後授權(PMA)
第8章:市場估算與預測:依最終用途分類,2021-2034年
- 主要趨勢
- 製藥公司
- 生技公司
- 醫療器材公司
第9章:市場估計與預測:依地區分類,2021-2034年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- Accell Clinical Research
- Charles River Laboratories
- Clinilabs
- Freyr
- Genpact
- ICON
- Labcorp
- NAMSA
- PAREXEL
- PharmaLex
- ProPharma
- Proventa
- Syneos Health
- Thermo Fisher Scientific
The Global Healthcare Regulatory Affairs Outsourcing Market was valued at USD 7.5 billion in 2024 and is estimated to grow at a CAGR of 7.6% to reach USD 15.7 billion by 2034.

The increasing complexity of global regulatory frameworks compelling pharmaceutical, biotechnology, and medical device companies to outsource compliance, documentation, and submission tasks to specialized partners. The market provides end-to-end solutions that help streamline regulatory processes, ensure adherence to international standards, and accelerate product approvals. Services include regulatory strategy formulation, dossier preparation, eCTD publishing, labeling management, lifecycle maintenance, and regulatory intelligence, all aimed at enhancing efficiency, safety, and patient outcomes. The rapid adoption of digital and cloud-based regulatory information management (RIM) systems, combined with AI-driven analytics, is driving demand for outsourcing providers skilled in managing electronic submissions and complex regulatory workflows. Furthermore, the growing number of global clinical trials, including adaptive and decentralized studies, is increasing regulatory burdens and prompting companies to rely on experienced external partners for submission planning, compliance tracking, and interactions with health authorities.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $7.5 Billion |
| Forecast Value | $15.7 Billion |
| CAGR | 7.6% |
In 2024, the product registration & clinical trial application segment held a 28.4% share. This segment's growth is fueled by increasing drug development activities and stringent regulatory requirements worldwide. Outsourcing is essential to navigate the complexity of submissions, ensuring compliance with agencies like the FDA, EMA, and PMDA, which continue to tighten guidelines to safeguard drug safety and efficacy.
The oncology segment held a 32.4% share in 2024 and is expected to reach USD 5.4 billion during 2025-2034. Rising global cancer incidence, increasing awareness, and early detection programs are driving demand for innovative oncology therapies. Companies are investing heavily in oncology drug development, creating complex, multi-region regulatory submissions. The high cost and long duration of oncology trials further encourage outsourcing to firms with specialized expertise in oncology regulations.
North America Healthcare Regulatory Affairs Outsourcing Market held a 46.5% share in 2024. The region's dominance stems from its robust pharmaceutical, biotechnology, and medical device industries, which require extensive regulatory support to comply with stringent frameworks. Evolving regulations from authorities such as the U.S. FDA and Health Canada, including guidelines on clinical trials, biologics, and post-market monitoring, have increased reliance on outsourcing partners.
Key players operating in the Global Healthcare Regulatory Affairs Outsourcing Market include Thermo Fisher Scientific, Clinilabs, Genpact, ICON, Syneos Health, Accell Clinical Research, Charles River Laboratories, Labcorp, NAMSA, PAREXEL, PharmaLex, ProPharma, and Proventa. Companies in the Healthcare Regulatory Affairs Outsourcing Market strengthen their presence by investing in specialized expertise, particularly for complex therapy areas like oncology and biologics. They develop integrated digital platforms for electronic submissions, AI-driven regulatory intelligence, and cloud-based RIM systems to streamline compliance workflows. Strategic partnerships with global pharmaceutical and biotech firms help expand service portfolios and geographic reach. Firms focus on regulatory consulting, dossier management, and lifecycle maintenance to enhance customer loyalty.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Services trends
- 2.2.3 Indication trends
- 2.2.4 Product stage trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing need to comply with regulatory requirements
- 3.2.1.2 Surging demand for faster approval process for breakthrough drugs and devices
- 3.2.1.3 Rising number of clinical trials
- 3.2.1.4 Increasing regulatory complexity
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Data security and privacy concerns
- 3.2.2.2 Lack of standardization
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of regulatory outsourcing for emerging markets
- 3.2.3.2 Rising demand for end-to-end RIM
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 LAMEA
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.1.1 Adoption of cloud-based regulatory submission and document management platforms
- 3.5.1.2 Implementation of AI and machine learning for regulatory intelligence and compliance monitoring
- 3.5.1.3 Integration of digital tools for global submission tracking and e-submissions
- 3.5.2 Emerging technologies
- 3.5.2.1 AI-enabled regulatory decision support and predictive compliance analytics
- 3.5.2.2 Blockchain for secure, transparent, and traceable regulatory documentation
- 3.5.1 Current technological trends
- 3.6 Future market trends
- 3.6.1 Regulatory milestone accelerating market entry
- 3.6.2 Increased R&D and investment activity
- 3.6.3 Enhanced regulatory compliance and validation
- 3.7 Investment and funding landscape
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Services, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Product registration & clinical trial application
- 5.3 Regulatory consulting/strategic services
- 5.4 Submission management
- 5.5 Legal representation
- 5.6 Regulatory writing & publishing
- 5.7 Other services
Chapter 6 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Neurology
- 6.4 Cardiology
- 6.5 Immunology
- 6.6 Other indications
Chapter 7 Market Estimates and Forecast, By Product Stage, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Preclinical
- 7.3 Clinical
- 7.4 Post market authorization (PMA)
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pharmaceutical companies
- 8.3 Biotechnology companies
- 8.4 Medical device companies
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Accell Clinical Research
- 10.2 Charles River Laboratories
- 10.3 Clinilabs
- 10.4 Freyr
- 10.5 Genpact
- 10.6 ICON
- 10.7 Labcorp
- 10.8 NAMSA
- 10.9 PAREXEL
- 10.10 PharmaLex
- 10.11 ProPharma
- 10.12 Proventa
- 10.13 Syneos Health
- 10.14 Thermo Fisher Scientific






