 |
市場調查報告書
商品編碼
1876646
產後出血管理器材市場機會、成長促進因素、產業趨勢分析及預測(2025-2034年)Postpartum Hemorrhage Management Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球產後出血管理設備市場價值為 9.586 億美元,預計到 2034 年將以 5.2% 的複合年成長率成長至 16 億美元。
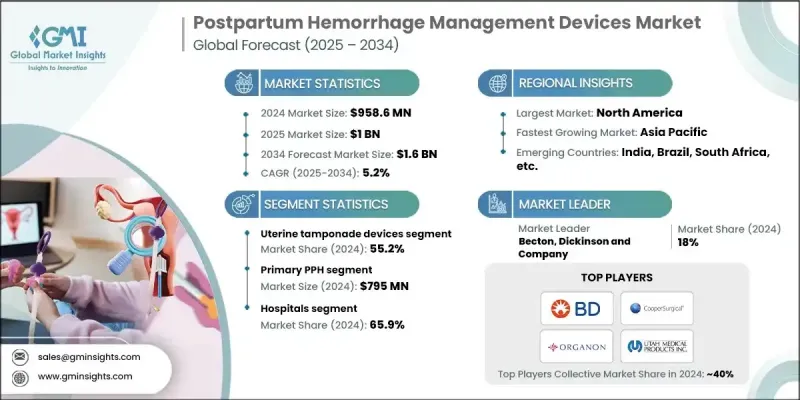
隨著全球產後出血發生率的上升和孕產婦健康意識的增強,產後出血管理產業持續發展。設備創新快速發展、緊急產科護理的日益普及以及旨在預防分娩併發症的教育宣傳活動不斷加強,也推動了市場需求的成長。這些努力透過擴大孕產婦護理技術的普及範圍、加強與醫療系統的合作以及提高整體應用率,為製造商創造了更有利的市場環境。人工智慧驅動的分析工具、數位連接、攜帶式設備和其他技術進步的日益融合,預計將在未來幾年加速產後出血管理設備的發展。產後出血管理設備旨在控制分娩後的過度出血,並有助於降低產科急症的孕產婦死亡率。由於子宮球囊系統的高效性、微創性和經證實的有效性,臨床團隊越來越依賴該系統。其良好的安全性和可靠的性能使其在全球醫療機構中的應用日益廣泛。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 9.586億美元 |
| 預測值 | 16億美元 |
| 複合年成長率 | 5.2% |
2024年,子宮填塞器械市佔率達到55.2%,這主要得益於子宮收縮無力、貧血等疾病的高發生率,以及政府為改善孕產婦健康狀況所採取的各項措施。這些器械透過施加子宮內壓力來控制嚴重出血,通常可以減少手術的必要性,並迅速穩定患者病情。
2024年,繼發性產後出血的市場規模預計為1.636億美元。繼發性產後出血是指在分娩後24小時至12週內發生的出血,通常與感染、組織殘留或傷口癒合延遲有關。這類出血往往需要影像檢查,並使用子宮收縮劑或手術治療。雖然繼發性產後出血不如原發性產後出血常見,但其預後更為複雜,凸顯了長期孕產婦健康規劃和預防保健策略的重要性。
2024年,美國產後出血管理器材市場規模預計將達3.308億美元。該國產後出血的高發生率持續推高了產品需求。由於監管力度強、醫院體系完善以及民眾對孕產婦健康的高度重視,美國仍是產後出血管理器械的主要應用國家之一。公共措施和私人投資正在擴大這些器材的普及範圍,並促進進一步的創新。
產後出血管理器械市場的主要參與者包括貝克頓·迪金森公司 (Becton, Dickinson and Company)、PREGNA INTERNATIONAL、CooperSurgical、SINAPI、CELOX MEDICAL、ORGANON、STERIMED、ZOEX NIASG、3rd Stone Design、UTAH MEDICAL PRODUCTS, INC. 和 MedGyn。參與產後出血管理器材市場競爭的公司正致力於採取多項策略性措施來提升其全球地位。許多公司正加大對先進產品工程的投資,以提高產品的可靠性、便攜性和病人安全性。各機構正在擴大研發項目,以推出能夠更快部署並降低干預風險的新型器械設計。加強與醫院和孕產婦保健網路的合作關係,以及拓展在已開發地區和新興地區的銷售管道,仍是各公司的首要任務。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 產業影響因素
- 成長促進因素
- 全球產後出血發生率不斷上升
- 加強對孕產婦死亡率的認知宣導活動
- 產後出血管理設備的技術進步
- 產業陷阱與挑戰
- 資源匱乏環境下的設備取得受限
- 新設備核准的監理延誤
- 機會
- 將人工智慧應用於預測性孕產婦健康分析
- 增加對孕產婦健康計畫的撥款
- 成長促進因素
- 成長潛力分析
- 監管環境
- 北美洲
- 歐洲
- 亞太地區
- 技術與創新格局
- 當前技術趨勢
- 新興技術
- 投資環境
- 2024年定價分析
- 報銷方案
- 永續性和環境考量
- 臨床證據與結果分析
- 波特的分析
- PESTEL 分析
- 差距分析
- 未來市場趨勢
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 公司市佔率分析
- 全球的
- 北美洲
- 歐洲
- 亞太地區
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 合作夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估算與預測:依產品類型分類,2021-2034年
- 主要趨勢
- 子宮填塞裝置
- 球囊填塞
- 真空誘導裝置
- 非氣動防震衣(NASG)
- 預填充注射系統
第6章:市場估計與預測:依病患類型分類,2021-2034年
- 主要趨勢
- 原發性產後出血
- 繼發性產後出血
第7章:市場估算與預測:依最終用途分類,2021-2034年
- 主要趨勢
- 醫院
- 孕產中心
- 門診手術中心
- 其他最終用途
第8章:市場估算與預測:依地區分類,2021-2034年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- MEA
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- 3rd Stone Design
- Becton, Dickinson and Company
- CELOX MEDICAL
- CooperSurgical
- MedGyn
- ORGANON
- PREGNA INTERNATIONAL
- SINAPI
- STERIMED
- UTAH MEDICAL PRODUCTS, INC.
- ZOEX NIASG
The Global Postpartum Hemorrhage Management Devices Market was valued at USD 958.6 million in 2024 and is estimated to grow at a CAGR of 5.2% to reach USD 1.6 billion by 2034.

The industry continues to advance as the worldwide occurrence of postpartum hemorrhage climbs and awareness of maternal health strengthens. Demand is also influenced by rapid progress in device innovation, growing use of emergency obstetric care, and heightened educational campaigns focused on preventing childbirth complications. These efforts are helping create a more supportive environment for manufacturers by expanding access to maternal care technologies, building stronger collaborations with healthcare systems, and improving overall adoption. Increasing integration of AI-driven analytical tools, digital connectivity, portable equipment, and other technological enhancements is expected to accelerate development over the coming years. Postpartum hemorrhage management devices are designed to control excessive bleeding after childbirth and contribute to reducing maternal mortality during obstetric emergencies. Clinical teams increasingly rely on uterine balloon systems due to their efficiency, minimally invasive nature, and proven effectiveness. Strong safety results and dependable performance continue to broaden their acceptance in healthcare facilities worldwide.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $958.6 Million |
| Forecast Value | $1.6 Billion |
| CAGR | 5.2% |
The uterine tamponade segment held a 55.2% share in 2024, supported by high rates of conditions such as uterine atony and anemia, as well as governmental measures aimed at improving maternal outcomes. These devices deliver internal uterine pressure to manage severe bleeding and can often reduce the need for surgical procedures while stabilizing patients rapidly.
The secondary postpartum hemorrhage segment was valued at USD 163.6 million in 2024. Defined as bleeding occurring more than 24 hours and up to 12 weeks after childbirth, this form of hemorrhage is generally associated with infection, retained tissue, or delayed healing. Diagnostic imaging and treatment using uterotonic medications or surgical options are often required. Although less common than primary postpartum hemorrhage, the secondary category is linked to more complex outcomes and highlights the importance of long-term maternal health planning and preventive-care strategies.
United States Postpartum Hemorrhage Management Devices Market generated USD 330.8 million in 2024. The country's high prevalence of postpartum hemorrhage continues to elevate product demand. The U.S. remains one of the leading adopters of PPH devices due to strong regulatory oversight, a widely developed hospital system, and extensive awareness of maternal health priorities. Public initiatives and private investment in maternal care are broadening access to these devices and stimulating further innovation.
Key Postpartum Hemorrhage Management Devices Market participants include Becton, Dickinson and Company, PREGNA INTERNATIONAL, CooperSurgical, SINAPI, CELOX MEDICAL, ORGANON, STERIMED, ZOEX NIASG, 3rd Stone Design, UTAH MEDICAL PRODUCTS, INC., and MedGyn. Companies competing in the postpartum hemorrhage management devices market are focusing on several strategic actions to enhance their global standing. Many firms are channeling investment toward advanced product engineering to improve reliability, portability, and patient safety. Organizations are expanding R&D programs to introduce new device designs that offer quicker deployment and reduced intervention risks. Strengthening relationships with hospitals and maternal care networks remains a priority, along with broadening distribution in both developed and emerging regions.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product type trends
- 2.2.3 Patient type trends
- 2.2.4 End Use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of postpartum hemorrhage globally
- 3.2.1.2 Increasing maternal mortality awareness campaigns
- 3.2.1.3 Technological advancements in PPH management devices
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Limited access to devices in low-resource settings
- 3.2.2.2 Regulatory delays for new device approvals
- 3.2.3 Opportunities
- 3.2.3.1 Integration of AI for predictive maternal health analytics
- 3.2.3.2 Increased funding for maternal health programs
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology and innovation landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Investment landscape
- 3.7 Pricing analysis, 2024
- 3.8 Reimbursement scenario
- 3.9 Sustainability & environmental considerations
- 3.10 Clinical evidence & outcomes analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
- 3.13 Gap analysis
- 3.14 Future market trends
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Uterine tamponade devices
- 5.2.1 Balloon tamponade
- 5.2.2 Vacuum-induced devices
- 5.3 Non-pneumatic anti-shock garments (NASG)
- 5.4 Prefilled injection system
Chapter 6 Market Estimates and Forecast, By Patient Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Primary PPH
- 6.3 Secondary PPH
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Maternity & birthing centers
- 7.4 Ambulatory surgical centers
- 7.5 Other End Use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 MEA
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 3rd Stone Design
- 9.2 Becton, Dickinson and Company
- 9.3 CELOX MEDICAL
- 9.4 CooperSurgical
- 9.5 MedGyn
- 9.6 ORGANON
- 9.7 PREGNA INTERNATIONAL
- 9.8 SINAPI
- 9.9 STERIMED
- 9.10 UTAH MEDICAL PRODUCTS, INC.
- 9.11 ZOEX NIASG





