 |
市場調查報告書
商品編碼
1871275
生物分析檢測服務市場機會、成長促進因素、產業趨勢分析及預測(2025-2034年)Bioanalytical Testing Services Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球生物分析測試服務市場價值為 42 億美元,預計到 2034 年將以 8.2% 的複合年成長率成長至 93 億美元。
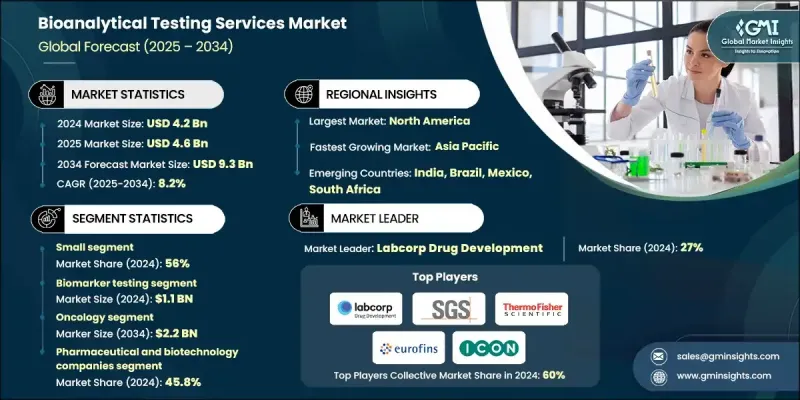
實驗室檢測外包趨勢的日益成長、生物分析技術的快速發展以及藥物研發和核准流程的不斷增加,共同推動了生物分析檢測服務的穩定成長。臨床試驗和研究活動的激增也促進了市場擴張。生物分析檢測服務涉及對包括血液、組織和尿液在內的生物樣本進行分析,以定量生物基質中的藥物、代謝物、生物標記和其他分析物。這些服務對於製藥和生物技術產業進行藥物動力學研究、生物等效性評估、方法驗證和臨床試驗支援至關重要。它們確保藥物的安全性、有效性和合規性,同時為藥效學研究和生物標記分析提供關鍵資料。對精確、可靠和高品質生物分析資料的需求不斷成長,並持續推動全球對這些服務的應用。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 42億美元 |
| 預測值 | 93億美元 |
| 複合年成長率 | 8.2% |
到2024年,小分子藥物市佔率將達到56%。小分子藥物在各個治療領域的持續普及推動了對小分子藥物測試需求的成長。小分子藥物結構相對簡單、監管途徑成熟且生產成本低廉,因此深受製藥公司青睞。此外,學名藥獲批數量的激增以及品牌小分子藥物專利的到期也加劇了生物等效性研究的需求。
預計到2024年,製藥和生物技術公司將佔據45.8%的市場。這些公司高度依賴生物分析檢測來支援複雜生物製劑、生物相似藥和標靶療法的研發,從而確保符合監管要求並確保資料的完整性。生物分析服務提供者與製藥或生技公司之間的合作正在迅速擴展,這不僅提高了營運效率,加快了藥物研發進程,也維持了高品質的檢測標準。
2024年,美國生物分析檢測服務市場規模預估達15億美元。推動市場成長的主要因素包括製藥和生物技術公司研發投入的增加,尤其是在生物製劑開發領域,以及與合約研究組織(CRO)和合約開發與生產組織(CDMO)的策略合作。北美生物分析檢測服務市場受益於先進的製藥和生物技術基礎設施、廣泛的研究活動以及大量需要專業檢測服務的藥物發現和開發項目。
全球生物分析檢測服務市場的主要公司包括:賽默飛世爾科技公司 (Thermo Fisher Scientific Inc.)、Intertek Group Plc、藥明康德 (WuXi AppTec)、ICON Plc、Pace Analytical Services LLC、BioAgilytix、Eurofins Scientific、Labcorp Drug、Face Analytical Services LLC、BioAgilytix、Eurofins Scientific、Labcorp Drug Development、Charles Riverynea. Generals. Health、Cambrex Corporation、Altasciences 和 LGC Limited。這些公司正透過多種策略舉措鞏固其市場地位。它們大力投資研發,以提升分析能力、提高靈敏度並擴大服務範圍。與製藥、生物技術和合約研究機構的策略合作與夥伴關係,有助於加速市場拓展並提升服務效率。併購則有助於整合技術專長、擴大地域覆蓋範圍並擴大營運規模。此外,各公司也致力於獲得監管部門的批准、認證和方法驗證,以提升信譽並吸引高階客戶。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 產業影響因素
- 成長促進因素
- 實驗室檢測服務外包趨勢日益成長
- 生物分析技術的不斷進步
- 藥物研發和核准流程的不斷發展
- 正在進行的研究活動和臨床試驗數量不斷增加
- 產業陷阱與挑戰
- 實驗室維護的複雜監管框架
- 先進分析儀器和技術成本高昂
- 市場機遇
- 擴大新興市場的臨床試驗
- 人工智慧與自動化在生物分析工作流程中的整合
- 成長促進因素
- 成長潛力分析
- 監管環境
- 技術進步
- 當前技術趨勢
- 新興技術
- 供應鏈分析
- 報銷方案
- 2024年定價分析
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估算與預測:依分子類型分類,2021-2034年
- 主要趨勢
- 小分子
- 大分子
第6章:市場估算與預測:依測試類型分類,2021-2034年
- 主要趨勢
- DMPK 檢測
- 生物標記檢測
- 病毒學檢測
- 體內病毒學檢測
- 體外病毒學檢測
- 血清學檢測
- 免疫原性檢測
- 其他測試類型
第7章:市場估計與預測:依應用領域分類,2021-2034年
- 主要趨勢
- 腫瘤學
- 神經病學
- 傳染病
- 胃腸病學
- 心臟病學
- 其他應用
第8章:市場估算與預測:依最終用途分類,2021-2034年
- 主要趨勢
- 製藥和生物技術公司
- 合約研究機構
- 研究和學術機構
- 其他最終用途
第9章:市場估計與預測:依地區分類,2021-2034年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- Altasciences
- BioAgilytix
- Cambrex Corporation
- Charles River Laboratories, Inc.
- Eurofins Scientific
- Frontage Labs
- ICON Plc
- Intertek Group Plc
- Labcorp Drug Development
- LGC Limited
- Pace Analytical Services LLC
- SGS Societe Generale de Surveillance SA
- Syneos Health
- Thermo Fisher Scientific Inc.
- WuXi AppTec
The Global Bioanalytical Testing Services Market was valued at USD 4.2 billion in 2024 and is estimated to grow at a CAGR of 8.2% to reach USD 9.3 billion by 2034.

The steady growth is fueled by the increasing trend of outsourcing laboratory testing, rapid advancements in bioanalytical technologies, and the rising volume of drug development and approval processes. The surge in clinical trials and research activities also supports market expansion. Bioanalytical testing services involve analyzing biological samples, including blood, tissue, and urine, to quantify drugs, metabolites, biomarkers, and other analytes in biological matrices. These services are crucial for the pharmaceutical and biotechnology industries to conduct pharmacokinetic studies, bioequivalence evaluations, method validations, and clinical trial support. They ensure drug safety, efficacy, and regulatory compliance while providing essential data for pharmacodynamic studies and biomarker analysis. Growing demand for precise, reliable, and high-quality bioanalytical data continues to drive the adoption of these services globally.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.2 Billion |
| Forecast Value | $9.3 Billion |
| CAGR | 8.2% |
The small molecule segment held a 56% share in 2024. The increasing demand for small molecule testing is driven by the continued prevalence of small molecule drugs in various therapeutic areas. Their relatively simpler chemical structures, established regulatory pathways, and cost-effective production make them favorable for pharmaceutical companies. Moreover, the surge in generic drug approvals and patent expirations for branded small-molecule drugs has escalated the need for bioequivalence studies.
The pharmaceutical and biotechnology companies segment held a 45.8% share in 2024. These organizations rely heavily on bioanalytical testing to support the development of complex biologics, biosimilars, and targeted therapies, ensuring regulatory compliance and robust data integrity. Collaborations between bioanalytical service providers and pharmaceutical or biotech companies are expanding rapidly, enhancing operational efficiency, accelerating drug development timelines, and maintaining high-quality testing standards.
United States Bioanalytical Testing Services Market was valued at USD 1.5 billion in 2024. Growth is driven by increased R&D investments by pharmaceutical and biotechnology firms, particularly in biologics development, and strategic partnerships with contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs). North America's bioanalytical testing services market benefits from advanced pharmaceutical and biotechnology infrastructure, extensive research activities, and a high number of drug discovery and development projects requiring specialized testing services.
Key companies in the Global Bioanalytical Testing Services Market include Thermo Fisher Scientific Inc., Intertek Group Plc, WuXi AppTec, ICON Plc, Pace Analytical Services LLC, BioAgilytix, Eurofins Scientific, Labcorp Drug Development, Charles River Laboratories, Inc., SGS Societe Generale de Surveillance SA, Frontage Labs, Syneos Health, Cambrex Corporation, Altasciences, and LGC Limited. Companies in the Global Bioanalytical Testing Services Market are strengthening their presence through several strategic approaches. They are investing heavily in research and development to enhance analytical capabilities, improve sensitivity, and expand service portfolios. Strategic collaborations and partnerships with pharmaceutical, biotechnology, and contract research organizations accelerate market reach and improve service efficiency. Mergers and acquisitions help consolidate technological expertise, expand geographical presence, and scale operations. Firms are also focusing on regulatory approvals, accreditation, and method validation to enhance credibility and attract high-profile clients.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Molecule type trends
- 2.2.3 Test type trend
- 2.2.4 Application trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increased trend of outsourcing laboratory testing services
- 3.2.1.2 Rising advancements in bioanalytical technology
- 3.2.1.3 Growing drug development and approval processes
- 3.2.1.4 Increasing volume of ongoing research activities and clinical trials
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Complex regulatory framework for maintaining laboratories
- 3.2.2.2 High cost of advanced analytical instruments and technologies
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of clinical trials in emerging markets
- 3.2.3.2 Integration of AI and automation in bioanalytical workflows
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Reimbursement scenario
- 3.8 Pricing analysis, 2024
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Molecule Type, 2021 - 2034 ($ Bn)
- 5.1 Key trends
- 5.2 Small molecule
- 5.3 Large molecule
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Bn)
- 6.1 Key trends
- 6.2 DMPK Testing
- 6.3 Biomarker testing
- 6.4 Virology testing
- 6.4.1 In-vivo virology testing
- 6.4.2 In-vitro virology testing
- 6.5 Serology testing
- 6.6 Immunogenicity testing
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Bn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Neurology
- 7.4 Infectious diseases
- 7.5 Gastroenterology
- 7.6 Cardiology
- 7.7 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Bn)
- 8.1 Key trends
- 8.2 Pharmaceutical and biotechnology companies
- 8.3 Contract research organizations
- 8.4 Research and academic institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Bn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Altasciences
- 10.2 BioAgilytix
- 10.3 Cambrex Corporation
- 10.4 Charles River Laboratories, Inc.
- 10.5 Eurofins Scientific
- 10.6 Frontage Labs
- 10.7 ICON Plc
- 10.8 Intertek Group Plc
- 10.9 Labcorp Drug Development
- 10.10 LGC Limited
- 10.11 Pace Analytical Services LLC
- 10.12 SGS Societe Generale de Surveillance SA
- 10.13 Syneos Health
- 10.14 Thermo Fisher Scientific Inc.
- 10.15 WuXi AppTec









