 |
市場調查報告書
商品編碼
1858967
藥物基因體學市場機會、成長促進因素、產業趨勢分析及預測(2025-2034年)Pharmacogenomics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球藥物基因組學市場價值為 66 億美元,預計到 2034 年將以 10.4% 的複合年成長率成長至 177 億美元。
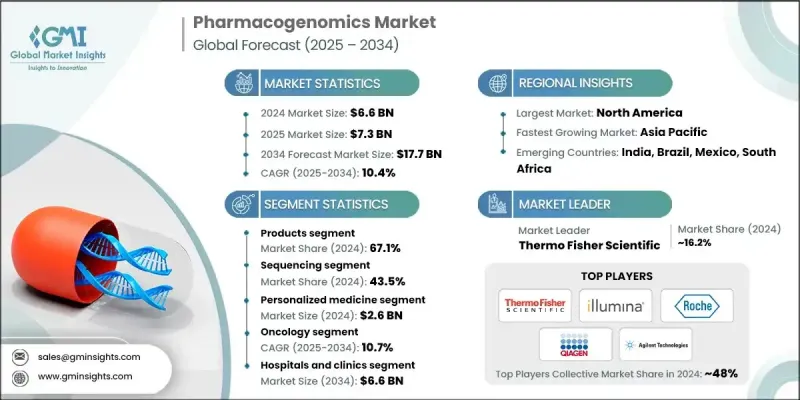
標靶治療需求的不斷成長以及基因組技術的持續發展推動了藥物基因組學的穩定成長。隨著全球醫療體係不斷推進個人化治療方案,藥物基因組學檢測在臨床和研究領域的應用日益廣泛。癌症、心血管疾病和傳染病等慢性疾病的沉重負擔進一步推動了基因組學在決策中發揮越來越重要的作用。藥物基因組學專注於了解個體的基因如何影響其藥物反應,使臨床醫生能夠最佳化劑量和治療方案,提高安全性和有效性。向數位化醫療解決方案的持續轉型,以及人工智慧驅動的臨床決策工具和真實世界資料分析的融合,不斷推動該領域的發展。市場的發展與個人化醫療的廣泛趨勢以及在提高治療效果的同時減少藥物不良反應的需求密切相關。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 66億美元 |
| 預測值 | 177億美元 |
| 複合年成長率 | 10.4% |
2024年,產品板塊佔據67.1%的市場佔有率,主要受個人化醫療工具需求成長的推動。該板塊涵蓋儀器、耗材以及各種基因檢測試劑盒和試劑,這些產品對臨床和研究應用至關重要。定序試劑盒、基於PCR的試劑、微陣列和其他診斷工具等細分領域廣泛應用於心臟病學、腫瘤學、精神病學和傳染病學等多個專科。治療決策中對精準解決方案的需求不斷成長,並持續推動藥物基因組學產品的需求。
2024年,個人化醫療市場規模預計將達26億美元。其快速成長歸功於先進基因組工具的普及、診斷技術的進步以及資料分析在常規醫療實踐中的應用。國家基因組學計畫正積極推動將藥物基因組學資料納入日常臨床實踐。監管機構透過批准相關生物標記來支持這項轉型,從而實現更安全的用藥和個人化治療。伴隨診斷和人工智慧技術的日益普及,也使得個人化醫療更具規模化優勢,並被更廣泛地接受。
2024年,北美藥物基因組學市佔率達到48.6%。美國和加拿大市場擴張得益於對基因組創新的高度重視、高昂的醫療保健支出以及先進的數位基礎設施。醫院和診所對基因組檢測和個人化治療的日益普及,得益於有利的報銷政策,從而推動了藥物基因組學檢測在臨床實踐中的廣泛應用。定序工具和人工智慧診斷技術的不斷進步正在改善臨床療效,並推動區域市場滲透。
藥物基因組學市場的領導者正透過策略性研發投資、合作以及產品多元化來擴大市場佔有率。例如,賽默飛世爾科技、安捷倫科技、Illumina 和 Qiagen 等公司致力於開發能夠實現更快、更精準基因分析的綜合試劑盒、試劑和定序平台。與醫療機構和研究機構的合作有助於這些公司共同開發滿足臨床需求的客製化解決方案。此外,企業也積極尋求併購,以增強技術實力並拓展地域覆蓋範圍。同時,各企業也高度重視法規核准和合規性,以確保產品的臨床相關性和市場准入。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 供應商格局
- 每個階段的價值增加
- 影響價值鏈的因素
- 產業影響因素
- 成長促進因素
- 增加研發投資
- 癌症發生率不斷上升
- 精準醫療方法的日益普及
- 藥物不良反應負擔日益加重
- 產業陷阱與挑戰
- 成本和報銷方面的挑戰
- 遺傳資料的複雜性解讀
- 市場機遇
- 基於微生物組和荷爾蒙標靶療法的創新
- 多基因檢測板和伴隨診斷的開發
- 成長促進因素
- 成長潛力分析
- 監管環境
- 未來市場趨勢
- 技術格局
- 目前技術
- 新興技術
- 專利分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 全球的
- 北美洲
- 歐洲
- 亞太地區
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估算與預測:依產品類型分類,2021-2034年
- 主要趨勢
- 產品
- 試劑盒和試劑
- 定序試劑盒和試劑
- PCR試劑盒和試劑
- 微陣列試劑盒和試劑
- 其他試劑盒和試劑
- 儀器和耗材
- 試劑盒和試劑
- 服務
第6章:市場估計與預測:依技術分類,2021-2034年
- 主要趨勢
- 定序
- PCR
- 微陣列
- 其他技術
第7章:市場估計與預測:依應用領域分類,2021-2034年
- 主要趨勢
- 個人化醫療
- 臨床研究
- 藥物發現與臨床前開發
- 其他應用
第8章:市場估計與預測:依疾病領域分類,2021-2034年
- 主要趨勢
- 腫瘤學
- 心血管疾病
- 神經系統疾病
- 傳染病
- 心理健康
- 其他疾病領域
第9章:市場估算與預測:依最終用途分類,2021-2034年
- 主要趨勢
- 醫院和診所
- 學術和研究機構
- 製藥和生物技術公司
- 合約研究組織(CRO)
- 其他最終用途
第10章:市場估計與預測:依地區分類,2021-2034年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第11章:公司簡介
- 23andMe
- Admera Health
- Agilent Technologies
- Becton, Dickinson and Company
- Bio-Rad Laboratories
- Charles River Laboratories
- Danaher
- Eurofins Scientific
- F. Hoffmann-La Roche
- Genelex
- Genomind
- Illumina
- Laboratory Corporation of America Holdings
- Novogene
- OneOme
- Qiagen
- Revvity
- Takara Bio
- Thermo Fisher Scientific
The Global Pharmacogenomics Market was valued at USD 6.6 billion in 2024 and is estimated to grow at a CAGR of 10.4% to reach USD 17.7 billion by 2034.

The steady growth is fueled by increasing demand for targeted therapies and the ongoing development of genomic technologies. As healthcare systems worldwide push for more personalized treatment approaches, pharmacogenomic testing is seeing wider adoption across clinical and research settings. The expanding role of genomics in decision-making is further propelled by the burden of chronic diseases such as cancer, cardiovascular diseases, and infectious conditions. Pharmacogenomics focuses on understanding how a person's genetic code influences their drug response, allowing clinicians to fine-tune dosages and treatment options for improved safety and efficacy. The ongoing shift toward digital health solutions, combined with the integration of AI-powered clinical decision tools and real-world data analytics, continues to advance the field. The market's progress is directly tied to the broader move toward personalized medicine and the need to reduce adverse drug reactions while enhancing therapeutic outcomes.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $6.6 Billion |
| Forecast Value | $17.7 Billion |
| CAGR | 10.4% |
In 2024, the products segment held a 67.1% share, driven by rising demand for tools supporting personalized care. This category includes instruments, consumables, and a wide range of genetic testing kits and reagents, which are essential for clinical and research use. Subsegments such as sequencing kits, PCR-based reagents, microarrays, and other diagnostic tools are widely used across various specialties, including cardiology, oncology, psychiatry, and infectious diseases. The increasing need for precision-driven solutions in therapeutic decision-making continues to boost demand for pharmacogenomic product offerings.
The personalized medicine segment generated USD 2.6 billion in 2024. Its rapid growth is attributed to the expansion of access to advanced genomic tools, improved diagnostics, and the integration of data analytics into routine care. Programs focused on national genomics initiatives are encouraging the inclusion of pharmacogenomic data into everyday clinical practice. Regulatory bodies are supporting this transition by approving relevant biomarkers that enable safer drug use and individualized therapy. Growing use of companion diagnostics and AI-powered technologies has made personalized medicine more scalable and widely accepted.
North America Pharmacogenomics Market held a 48.6% share in 2024. Market expansion in the U.S. and Canada is supported by a strong focus on genomic innovation, high healthcare expenditure, and advanced digital infrastructure. The increased adoption of genomic testing and personalized treatments across hospitals and clinics has been backed by favorable reimbursement policies, contributing to the widespread implementation of pharmacogenomic panels into clinical practice. Ongoing advancements in sequencing tools and AI-enabled diagnostics are enhancing clinical outcomes and driving regional market penetration.
Leading players in the Pharmacogenomics Market are expanding their market presence through strategic R&D investments, partnerships, and product diversification. Companies such as Thermo Fisher Scientific, Agilent Technologies, Illumina, and Qiagen are focused on developing comprehensive kits, reagents, and sequencing platforms that enable faster and more accurate genetic profiling. Collaborations with healthcare providers and research institutions help these firms co-develop custom solutions tailored to clinical needs. Mergers and acquisitions are being pursued to strengthen technological capabilities and widen geographic reach. Additionally, players are emphasizing regulatory approvals and compliance to ensure clinical relevance and market access.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumption and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Offering trends
- 2.2.3 Technology trends
- 2.2.4 Application trends
- 2.2.5 Disease area trends
- 2.2.6 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing research and development investments
- 3.2.1.2 Increasing prevalence of cancer
- 3.2.1.3 Increasing adoption of precision medicine approaches
- 3.2.1.4 Rising burden of adverse drug reactions
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Cost and reimbursement challenges
- 3.2.2.2 Complexity and interpretation of genetic data
- 3.2.3 Market opportunities
- 3.2.3.1 Innovation in microbiome-based and hormone-targeted therapies
- 3.2.3.2 Development of multi-gene panels and companion diagnostics
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.5 Future market trends
- 3.6 Technological landscape
- 3.6.1 Current technologies
- 3.6.2 Emerging technologies
- 3.7 Patent analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.2.4 Asia Pacific
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Offerings, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Products
- 5.2.1 Kits and reagents
- 5.2.1.1 Sequencing kits and reagents
- 5.2.1.2 PCR kits and reagents
- 5.2.1.3 Microarray kits and reagents
- 5.2.1.4 Other kits and reagents
- 5.2.2 Instrument and consumables
- 5.2.1 Kits and reagents
- 5.3 Services
Chapter 6 Market Estimates and Forecast, By Technology, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Sequencing
- 6.3 PCR
- 6.4 Microarray
- 6.5 Other technologies
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Personalized medicine
- 7.3 Clinical research
- 7.4 Drug discovery and preclinical development
- 7.5 Other applications
Chapter 8 Market Estimates and Forecast, By Disease Area, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Oncology
- 8.3 Cardiovascular diseases
- 8.4 Neurological diseases
- 8.5 Infectious diseases
- 8.6 Mental health
- 8.7 Other disease areas
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospitals and clinics
- 9.3 Academic and research institutions
- 9.4 Pharmaceutical and biotechnology companies
- 9.5 Contract research organization (CROs)
- 9.6 Other end use
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 Japan
- 10.4.3 India
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 23andMe
- 11.2 Admera Health
- 11.3 Agilent Technologies
- 11.4 Becton, Dickinson and Company
- 11.5 Bio-Rad Laboratories
- 11.6 Charles River Laboratories
- 11.7 Danaher
- 11.8 Eurofins Scientific
- 11.9 F. Hoffmann-La Roche
- 11.10 Genelex
- 11.11 Genomind
- 11.12 Illumina
- 11.13 Laboratory Corporation of America Holdings
- 11.14 Novogene
- 11.15 OneOme
- 11.16 Qiagen
- 11.17 Revvity
- 11.18 Takara Bio
- 11.19 Thermo Fisher Scientific












