 |
市場調查報告書
商品編碼
1858844
RNA干擾療法市場機會、成長促進因素、產業趨勢分析及預測(2025-2034年)RNA Interference Therapeutics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球 RNA 干擾療法市場價值為 27 億美元,預計到 2034 年將以 15.4% 的複合年成長率成長至 112 億美元。
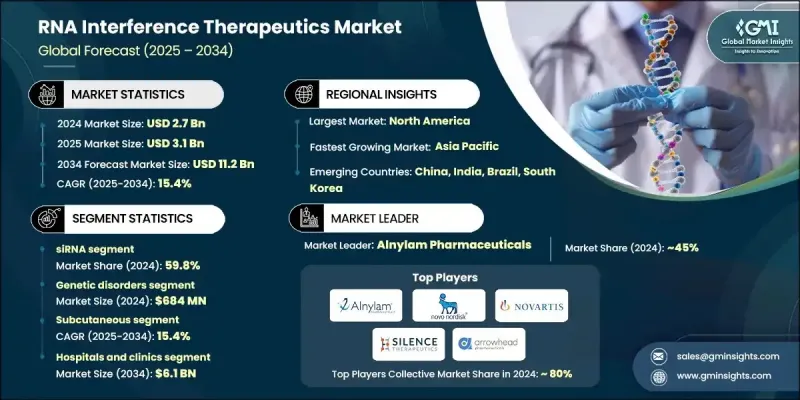
基於RNAi的療法日益重要,這源於基因研究的蓬勃發展、慢性疾病負擔的加重以及罕見遺傳疾病診斷率的上升。這些療法透過沉默疾病相關基因發揮作用,提供了一種精準的標靶治療方法。技術創新,特別是脂質奈米顆粒和偶聯系統等遞送方法的進步,顯著提高了RNAi療法的安全性和有效性。這拓寬了其臨床應用範圍,並促進了生物製藥產業的合作。 RNAi療法拓展至以往難以觸及的治療領域,尤其是在肝臟以外的領域,標誌著其臨床應用發生了關鍵性轉變。個人化醫療、基因編輯技術的進步以及基因療法在國際上日益成長的勢頭,都是推動市場發展的關鍵因素。有利的監管環境,特別是針對孤兒藥研發的監管政策,提供了加速核准和更長的專利保護期,使RNAi領域對製藥投資更具吸引力。在公部門和私部門的大力支持下,市場持續發展,推動RNAi從小眾科學走向主流療法。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 27億美元 |
| 預測值 | 112億美元 |
| 複合年成長率 | 15.4% |
2024年,小干擾RNA(siRNA)市佔率達到59.8%,預計2034年將達到68億美元,年複合成長率(CAGR)為15.5%。 siRNA療法因其基因沉默的精準性和較低的脫靶風險,處於RNAi技術發展的前沿。更先進的遞送技術以及對基因表現模式更深入的理解,使得siRNA的應用範圍擴展到肝臟以外的新治療標靶。 siRNA能夠調節心血管疾病、代謝性疾病和罕見遺傳性疾病中的基因表達,這極大地促進了其快速成長。在更廣泛的RNAi市場中,siRNA仍然是臨床應用最成熟、科學驗證最充分的領域。
2024年,遺傳性疾病領域創造了6.84億美元的市場規模。這一主導地位歸功於siRNA療法能夠精準靶向導致各種遺傳疾病的基因。這些療法在治療以往缺乏有效治療手段的疾病方面具有巨大潛力。 siRNA療法在治療罕見疾病和遺傳性疾病方面已證實有效,這支撐了該應用領域持續的臨床拓展和商業發展。
2024年,北美RNA干擾療法市場佔45.7%的比重。這一領先地位主要得益於先進的醫療基礎設施、高度發展的研究生態系統以及對創新療法的早期應用。美國國立衛生研究院等主要機構的大量資金投入,以及主要製藥企業和合約研發生產機構(CDMO)的積極參與,加速了該地區的藥物研發進程。對精準療法日益成長的需求以及遺傳疾病發病率的上升,進一步鞏固了該地區在該領域的領先地位。
全球RNA干擾療法市場的主要貢獻者包括Silence Therapeutics、Sirnaomics、Arrowhead Pharmaceuticals、Alnylam Pharmaceuticals、諾華、Creative Biogene、Arbutus Biopharma、OliX Pharmaceuticals、Benitec Biopharma、賽諾菲、諾和諾德和Atalanta Therapeutics。為了在RNAi療法市場中佔據更有利的地位,領先企業正大力推動策略合作、技術共享和研發投入。生物技術創新者與大型製藥公司之間的合作能夠加速藥物研發,獲得先進的給藥系統,並擴大市場覆蓋範圍。各公司也優先考慮產品線多元化,以涵蓋常見疾病和罕見疾病的療法。透過獲得孤兒藥資格和快速核准通道,企業可以獲得競爭優勢,例如延長獨佔期和縮短上市時間。
目錄
第1章:方法論與範圍
第2章:執行概要
第3章:行業洞察
- 產業生態系分析
- 供應商格局
- 每個階段的價值增加
- 影響價值鏈的因素
- 產業影響因素
- 成長促進因素
- 遺傳性疾病盛行率不斷上升
- RNA遞送技術的進步
- 以RNAi為基礎的研究投入不斷增加
- 核准RNAi療法用於治療慢性病和罕見疾病
- 產業陷阱與挑戰
- 有效遞送RNAi分子的挑戰
- RNAi療法研發成本高昂
- 市場機遇
- RNAi在神經病學和神經退化性疾病的應用
- 針對遺傳疾病的個人化RNAi療法
- 成長促進因素
- 成長潛力分析
- 監管環境
- 北美洲
- 美國
- 加拿大
- 歐洲
- 亞太地區
- 北美洲
- 技術格局
- 當前技術趨勢
- 新興技術
- 管道分析
- 未來市場趨勢
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估算與預測:依產品類型分類,2021-2034年
- 主要趨勢
- 小干擾RNA(siRNA)
- 微小RNA(miRNA)
- 短髮夾RNA(shRNA)
- 其他產品類型
第6章:市場估算與預測:依應用領域分類,2021-2034年
- 主要趨勢
- 癌症
- 遺傳性疾病
- 心血管疾病
- 病毒感染
- 神經退化性疾病
- 眼科疾病
- 呼吸系統疾病
- 其他應用
第7章:市場估計與預測:依給藥途徑分類,2021-2034年
- 主要趨勢
- 靜脈注射(IV)
- 皮下(SC)
- 其他給藥途徑
第8章:市場估算與預測:依最終用途分類,2021-2034年
- 主要趨勢
- 醫院和診所
- 研究實驗室
- 其他最終用途
第9章:市場估計與預測:依地區分類,2021-2034年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- Alnylam Pharmaceuticals
- Arbutus Biopharma
- Arrowhead Pharmaceuticals
- Atalanta Therapeutics
- Benitec Biopharma
- Creative Biogene
- Novartis
- Novo Nordisk
- OliX Pharmaceuticals
- Sanofi
- Silence Therapeutics
- Sirnaomics
The Global RNA Interference Therapeutics Market was valued at USD 2.7 billion in 2024 and is estimated to grow at a CAGR of 15.4% to reach USD 11.2 billion by 2034.

The growing importance of RNAi-based therapies stems from a surge in genetic research, the rising burden of chronic diseases, and increasing diagnoses of rare genetic disorders. These therapies work by silencing disease-related genes, offering a precise and targeted approach to treatment. Technological innovations, particularly in delivery methods like lipid nanoparticles and conjugate systems, have significantly enhanced the safety and effectiveness of RNAi therapies. This has widened the scope for clinical application and encouraged collaborations within the biopharmaceutical industry. The expansion of RNAi into therapeutic areas previously difficult to reach, especially beyond the liver, marks a pivotal shift in its clinical utility. Personalized medicine, advancements in gene editing, and growing international momentum for gene therapies are key contributors to the market's momentum. Favorable regulatory conditions, especially those geared toward orphan drug development, offer accelerated pathways and longer exclusivity, making the RNAi space more attractive for pharmaceutical investments. The market continues to evolve with strong backing from both public and private sectors, supporting the transition of RNAi from niche science to mainstream therapy.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.7 Billion |
| Forecast Value | $11.2 Billion |
| CAGR | 15.4% |
The small interfering RNA (siRNA) segment held a 59.8% share in 2024 and is projected to reach USD 6.8 billion by 2034, driven by a CAGR of 15.5%. siRNA therapies are at the forefront of RNAi advancements due to their gene-silencing precision and lower off-target risks. The development of more sophisticated delivery technologies and a deeper understanding of gene expression patterns has enabled expansion into new therapeutic targets outside the liver. The ability of siRNA to modulate gene expression in cardiovascular, metabolic, and rare inherited disorders is contributing significantly to its rapid growth. This segment remains the most clinically mature and scientifically validated within the broader RNAi market.
The genetic disorders segment generated USD 684 million in 2024. This dominance is attributed to the precision of siRNA therapies in targeting genes responsible for various inherited conditions. These therapies hold strong potential for addressing diseases that previously lacked effective treatment options. The proven efficacy of siRNA-based solutions in treating rare and genetically rooted conditions supports continued clinical expansion and commercial interest in this application area.
North America RNA Interference Therapeutics Market held a 45.7% share in 2024. This leadership is primarily driven by a combination of advanced healthcare infrastructure, a highly developed research ecosystem, and early adoption of innovative treatments. Significant funding from major institutions like the National Institutes of Health, along with active participation from major pharmaceutical players and CDMOs, has accelerated drug development in the region. The growing demand for precision-based therapeutics and rising rates of genetic diseases further support regional dominance in this space.
Key companies contributing to the Global RNA Interference Therapeutics Market include Silence Therapeutics, Sirnaomics, Arrowhead Pharmaceuticals, Alnylam Pharmaceuticals, Novartis, Creative Biogene, Arbutus Biopharma, OliX Pharmaceuticals, Benitec Biopharma, Sanofi, Novo Nordisk, and Atalanta Therapeutics. To establish a stronger presence in the RNAi therapeutics market, leading firms are focusing heavily on strategic partnerships, technology sharing, and R&D investments. Collaborations between biotech innovators and large pharmaceutical firms enable rapid drug development, access to advanced delivery systems, and broader market reach. Companies are also prioritizing pipeline diversification to include therapies targeting both common and rare diseases. By securing orphan drug status and fast-track regulatory designations, firms gain competitive advantages such as extended exclusivity and reduced time-to-market.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product type trends
- 2.2.3 Application trends
- 2.2.4 Route of administration trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of genetic disorders
- 3.2.1.2 Advancements in RNA delivery technologies
- 3.2.1.3 Rising investment in RNAi-based research
- 3.2.1.4 Approval of RNAi therapeutics for chronic and rare diseases
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Challenges in effective delivery of RNAi molecules
- 3.2.2.2 High cost of RNAi therapeutics development
- 3.2.3 Market opportunities
- 3.2.3.1 RNAi applications in neurology and neurodegenerative diseases
- 3.2.3.2 Personalized RNAi therapies for genetic diseases
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.1.1 U.S.
- 3.4.1.2 Canada
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.1 North America
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Pipeline analysis
- 3.7 Future market trends
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Small interfering RNA (siRNA)
- 5.3 MicroRNA (miRNA)
- 5.4 Short hairpin RNA (shRNA)
- 5.5 Other product types
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Cancer
- 6.3 Genetic disorders
- 6.4 Cardiovascular diseases
- 6.5 Viral infections
- 6.6 Neurodegenerative diseases
- 6.7 Ophthalmic disorders
- 6.8 Respiratory disorders
- 6.9 Other applications
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Intravenous (IV)
- 7.3 Subcutaneous (SC)
- 7.4 Other route of administration
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals and clinics
- 8.3 Research laboratories
- 8.4 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Alnylam Pharmaceuticals
- 10.2 Arbutus Biopharma
- 10.3 Arrowhead Pharmaceuticals
- 10.4 Atalanta Therapeutics
- 10.5 Benitec Biopharma
- 10.6 Creative Biogene
- 10.7 Novartis
- 10.8 Novo Nordisk
- 10.9 OliX Pharmaceuticals
- 10.10 Sanofi
- 10.11 Silence Therapeutics
- 10.12 Sirnaomics










