 |
市場調查報告書
商品編碼
1844317
幽門螺旋桿菌檢測市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Helicobacter Pylori Testing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球幽門螺旋桿菌檢測市場價值為 5.178 億美元,預計將以 7.1% 的複合年成長率成長,到 2034 年達到 10 億美元。
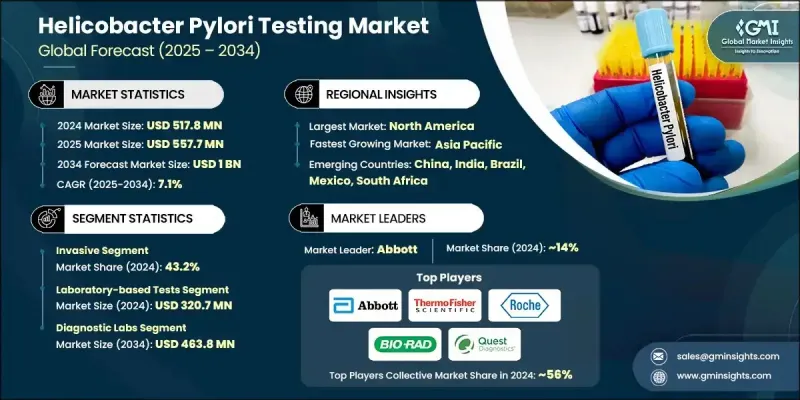
胃病患病率的上升、老年人口的增加以及人們對非侵入性診斷方法日益成長的興趣推動了這一行業的穩定成長。臨床診斷領域正在發生重大轉變,世界各地的醫療保健系統都致力於透過先進的檢測工具進行早期疾病檢測。隨著胃腸道疾病的日益普遍,對精準且患者友善的檢測的需求也日益成長。同時,人們對早期檢測的認知和偏好不斷提升,促使醫院和診斷中心擴大其幽門螺旋桿菌檢測能力。即時檢測因其快速、便捷且在各種醫療環境中易於獲取而受到廣泛關注。這些設備使臨床醫生能夠快速獲得結果,從而改善患者的治療效果並縮短治療時間。此外,分子診斷和數位健康工具(包括基於人工智慧的結果解讀和遠端檢測平台)的創新正在顯著拓寬市場可及性並提高臨床精準度。這些因素的共同作用,正在將幽門螺旋桿菌檢測領域塑造成全球胃腸道醫療診斷的關鍵領域。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 5.178億美元 |
| 預測值 | 10億美元 |
| 複合年成長率 | 7.1% |
侵入性檢測領域在2024年佔據了43.2%的市場佔有率,這得益於其透過組織活體組織切片和顯微鏡分析實現的卓越診斷準確性。這些方法能夠可靠地洞察細菌感染及其相關的胃部異常,尤其是在高風險或複雜病例中。當需要精確和詳細的病理學檢查時,臨床醫生仍然依賴侵入性操作,這進一步強化了其在全面診斷和癌症篩檢中的關鍵作用。
2024年,實驗室檢測細分市場收入達3.207億美元。該細分市場憑藉其提供的診斷資訊高度可靠且深度豐富而保持領先地位。組織學分析、培養和血清學檢測等實驗室檢測對於鑑定細菌菌株、確定感染階段和評估抗生素抗藥性至關重要。幽門螺旋桿菌相關胃腸道疾病(例如潰瘍和慢性胃炎)的發生率不斷上升,持續推動醫院和獨立實驗室對精準實驗室診斷的需求。
2024年,北美幽門螺旋桿菌檢測市場佔據34.6%的市場佔有率,這得益於該地區完善的醫療基礎設施和日益成長的胃腸道疾病負擔。強大的診斷實驗室、日益提升的臨床意識以及早期篩檢實踐,促進了檢測的廣泛應用。此外,該地區還受益於對先進診斷技術的持續投資以及非侵入性解決方案的日益普及,從而支持了美國和加拿大市場的持續成長。
全球幽門螺旋桿菌檢測市場的主要參與者包括雅培、生物梅里埃、賽默飛世爾科技、Bio-Rad Laboratories、Meridian Biosciences、Quidel Corporation、Gulf Coast Scientific、CERTEST、Coris BioConcept、Quest Diagnostics、羅氏、Cardinal Health 和 BIOHIT。幽門螺旋桿菌檢測市場的公司正在採取多方面的策略來鞏固其市場地位。領先的製造商正在優先進行研發投資,以開發具有更短週轉時間和更廣泛臨床應用的高靈敏度診斷試劑盒。許多公司正在擴展其非侵入性檢測產品組合,以滿足對患者友善診斷日益成長的需求。與醫院、診斷連鎖店和研究機構的策略合作夥伴關係使公司能夠擴大分銷網路並將檢測解決方案整合到主流臨床工作流程中。
目錄
第1章:方法論與範圍
第 2 章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 供應商格局
- 每個階段的增值
- 影響價值鏈的因素
- 產業衝擊力
- 成長動力
- 胃潰瘍盛行率上升
- 老年人口風險不斷增加
- 對即時檢測設備的需求不斷成長
- 非侵入性幽門螺旋桿菌檢測的採用率不斷上升
- 產業陷阱與挑戰
- 缺乏熟練的侵入性檢測專業人員
- 缺乏對幽門螺旋桿菌感染的認知
- 市場機會
- 分子檢測的技術創新
- 家庭和自我檢測試劑盒
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 當前的技術趨勢
- 採用非侵入性檢測(尿素呼氣、糞便抗原、血清學)
- 自動化和高通量診斷系統
- 與實驗室資訊系統(LIS)整合
- 提高檢測的敏感度和特異性
- 新興技術
- 攜帶式幽門螺旋桿菌檢測試劑盒
- 基於分子和PCR的精確檢測
- 用於活體組織切片和組織學分析的人工智慧和機器學習
- 同時檢測病原體的多重檢測平台
- 當前的技術趨勢
- 未來市場趨勢
- 非侵入式家用幽門螺旋桿菌檢測試劑盒的採用率不斷提高
- 擴展人工智慧驅動的診斷工具,實現更快、更準確的檢測
- 數位健康平台與遠距醫療在測試監控方面的整合日益加強
- 市場進入策略
- 定價分析
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 全球的
- 北美洲
- 歐洲
- 亞太地區
- 拉丁美洲和中東
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與協作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:按測試類型,2021 - 2034 年
- 主要趨勢
- 侵入性
- 快速尿素酶試驗
- 組織學
- 惠普文化
- 非侵入性
- 血清學檢測
- 尿素呼氣試驗
- 糞便抗原檢測
第6章:市場估計與預測:依方法,2021 - 2034 年
- 主要趨勢
- 實驗室測試
- 即時檢驗 (POC)
第7章:市場估計與預測:依最終用途,2021 - 2034
- 主要趨勢
- 診斷實驗室
- 醫院
- 診所
- 其他最終用途
第8章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- Abbott
- BIOHIT
- bioMerieux
- Bio-Rad Laboratories
- Cardinal Health
- CERTEST
- Coris BioConcept
- Gulf Coast Scientific
- Meridian Biosciences
- Quest Diagnostics
- Quidel Corporation
- Roche
- Thermo Fisher Scientific
The Global Helicobacter Pylori Testing Market was valued at USD 517.8 million in 2024 and is estimated to grow at a CAGR of 7.1% to reach USD 1 billion by 2034.

The steady growth is driven by the increasing prevalence of gastric disorders, a rise in the elderly population, and growing interest in non-invasive diagnostic approaches. A major shift is underway in clinical diagnostics, with healthcare systems across the world focusing on early disease detection through advanced testing tools. As gastrointestinal diseases become more common, the demand for accurate and patient-friendly tests is climbing. Simultaneously, rising awareness and preference for early detection are influencing hospitals and diagnostic centers to expand their H. pylori testing capabilities. Point-of-care testing is also gaining widespread traction due to its speed, convenience, and accessibility in a variety of healthcare settings. These devices enable clinicians to deliver rapid results, which enhances patient outcomes and treatment timelines. Additionally, innovations in molecular diagnostics and digital health tools including AI-based result interpretation and remote testing platforms are significantly broadening market accessibility and improving clinical precision. Combined, these elements are shaping the helicobacter pylori testing space into a key sector of gastrointestinal healthcare diagnostics worldwide.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $517.8 Million |
| Forecast Value | $1 Billion |
| CAGR | 7.1% |
The invasive testing segment captured 43.2% share in 2024, due to its superior diagnostic accuracy achieved through tissue biopsies and microscopic analysis. These methods offer reliable insights into bacterial infection and associated gastric abnormalities, particularly in high-risk or complicated cases. Clinicians continue to rely on invasive procedures when precision and detailed pathology are required, reinforcing their critical role in comprehensive diagnosis and cancer screening.
The laboratory-based tests segment generated USD 320.7 million in 2024. This segment holds its lead due to the high reliability and depth of diagnostic information provided. Laboratory tests like histological analysis, cultures, and serological assays are essential for identifying bacterial strains, determining infection stages, and assessing antibiotic resistance. Rising rates of H. pylori-associated gastrointestinal conditions such as ulcers and chronic gastritis continue to fuel the demand for precise lab-based diagnostics in both hospital and independent lab environments.
North American Helicobacter Pylori Testing Market held 34.6% share in 2024, driven by the region's well-established healthcare infrastructure and increasing gastrointestinal disease burden. Strong presence of diagnostic laboratories, growing clinical awareness, and early screening practices contribute to widespread test adoption. The region also benefits from ongoing investments in advanced diagnostics and rising uptake of non-invasive solutions, supporting sustained market growth across the US and Canada.
Key players actively competing in the Global Helicobacter Pylori Testing Market include Abbott, bioMerieux, Thermo Fisher Scientific, Bio-Rad Laboratories, Meridian Biosciences, Quidel Corporation, Gulf Coast Scientific, CERTEST, Coris BioConcept, Quest Diagnostics, Roche, Cardinal Health, and BIOHIT. Companies in the helicobacter pylori testing market are adopting multifaceted strategies to reinforce their market presence. Leading manufacturers are prioritizing R&D investments to develop high-sensitivity diagnostic kits with shorter turnaround times and broader clinical utility. Many firms are expanding their non-invasive testing portfolios to align with rising demand for patient-friendly diagnostics. Strategic partnerships with hospitals, diagnostic chains, and research institutions allow companies to widen distribution networks and integrate testing solutions into mainstream clinical workflows.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Test type trends
- 2.2.3 Method trends
- 2.2.4 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rise in prevalence of gastric ulcer
- 3.2.1.2 Increasing geriatric population at risk
- 3.2.1.3 Growing demand for point-of-care testing devices
- 3.2.1.4 Rising adoption of non-invasive H. pylori testing
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of skilled professionals for invasive testing
- 3.2.2.2 Lack of awareness regarding H. pylori infection
- 3.2.3 Market opportunities
- 3.2.3.1 Technological innovations in molecular testing
- 3.2.3.2 Home-based and self-testing kits
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.1.1 Non-invasive testing adoption (urea breath, stool antigen, serology)
- 3.5.1.2 Automation and high-throughput diagnostic systems
- 3.5.1.3 Integration with laboratory information systems (LIS)
- 3.5.1.4 Improved sensitivity and specificity of assays
- 3.5.2 Emerging technologies
- 3.5.2.1 Point-of-Care (POC) portable H. pylori detection kits
- 3.5.2.2 Molecular and PCR-based assays for precise detection
- 3.5.2.3 AI and machine learning for biopsy and histology analysis
- 3.5.2.4 Multiplex testing platforms for simultaneous pathogen detection
- 3.5.1 Current technological trends
- 3.6 Future market trends
- 3.6.1 Increased adoption of non-invasive home-based H. pylori testing kits
- 3.6.2 Expansion of AI-driven diagnostic tools for faster and more accurate detection
- 3.6.3 Growing integration of digital health platforms and telemedicine for test monitoring
- 3.7 Go-to-market strategies
- 3.8 Pricing analysis
- 3.9 GAP analysis
- 3.10 Porter’s analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.2.4 Asia Pacific
- 4.2.5 LAMEA
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Invasive
- 5.2.1 Rapid urease test
- 5.2.2 Histology
- 5.2.3 HP culture
- 5.3 Non-invasive
- 5.3.1 Serologic test
- 5.3.2 Urea breath test
- 5.3.3 Stool/fecal antigen test
Chapter 6 Market Estimates and Forecast, By Method, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Laboratory based tests
- 6.3 Point of care (POC) tests
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Diagnostic labs
- 7.3 Hospitals
- 7.4 Clinics
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott
- 9.2 BIOHIT
- 9.3 bioMerieux
- 9.4 Bio-Rad Laboratories
- 9.5 Cardinal Health
- 9.6 CERTEST
- 9.7 Coris BioConcept
- 9.8 Gulf Coast Scientific
- 9.9 Meridian Biosciences
- 9.10 Quest Diagnostics
- 9.11 Quidel Corporation
- 9.12 Roche
- 9.13 Thermo Fisher Scientific










