 |
市場調查報告書
商品編碼
1801909
胰臟癌診斷市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Pancreatic Cancer Diagnostic Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球胰臟癌診斷市場規模達25億美元,預計到2034年將以7.2%的複合年成長率成長,達到50億美元。這一成長主要得益於全球胰腺癌發病率的上升、醫療基礎設施投資的不斷增加以及診斷技術的持續進步。隨著人們對早期檢測認知的提高,以及醫療保健系統對更快診斷和更佳療效的重視,先進診斷工具的採用也隨之加速。這些工具——從分子診斷到高解析度成像和基於生物標記的檢測——在癌症的早期發現中發揮關鍵作用,尤其是在治療效果最佳的無症狀階段。
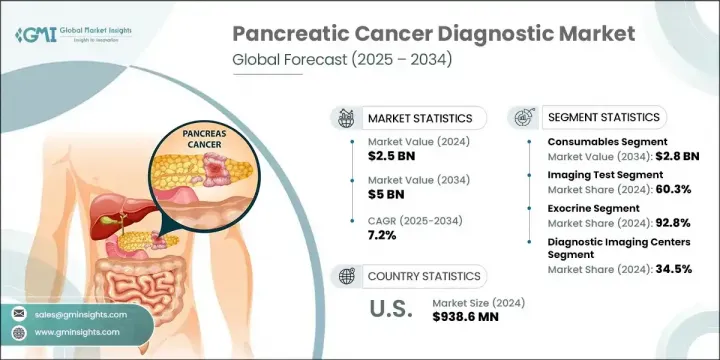
各種創新技術持續重塑診斷格局,人工智慧整合成像、分子分析和液體活體組織切片技術提升了診斷的準確性、效率和安全性。此外,個人化醫療和以患者為中心的護理的推動也凸顯了早期診斷的重要性。在工作流程效率提升和微創手術的支持下,臨床應用的擴展已使胰臟癌診斷成為現代腫瘤學實踐中不可或缺的一部分。人們日益成長的認知度,加上對及時精準診斷日益成長的需求,正在為未來十年的市場擴張創造有利條件。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 25億美元 |
| 預測值 | 50億美元 |
| 複合年成長率 | 7.2% |
2024年,耗材市場規模達14億美元,預估到2034年將達28億美元,複合年成長率為7%。該領域的強勁表現主要得益於試劑、檢測試劑盒和檢測材料在診斷領域的持續使用。隨著液體活體組織切片、分子診斷和免疫測定需求的不斷成長,對能夠確保檢測一致性、相容性和可重複性的可靠耗材的需求也日益成長。耗材在實現快速且可重複的檢測方面發揮著重要作用,使其成為臨床實驗室和醫院環境中不可或缺的一部分。
2024年,外分泌胰腺癌佔了92.8%的市場佔有率,這得益於各種外分泌胰腺癌的高發病率,包括鱗狀細胞癌、腺鱗癌、膠體癌和腺癌。由於大多數胰臟癌病例都屬於此類,醫療保健提供者優先考慮先進的診斷解決方案,以實現早期發現、準確分期和簡化治療計劃。自動化病變檢測、冷凍活體組織切片創新和人工智慧輔助診斷等新興技術正透過更快、更精準的結果來推動改善患者預後。
2024年,診斷影像中心市佔率達34.5%,這得益於其專業的基礎設施以及利用MRI、CT和內視鏡超音波(EUS)等先進設備進行快速高解析度掃描的能力。這些中心在促進胰臟癌的準確診斷、分期和監測方面繼續發揮至關重要的作用。其一體化的工作流程和先進的診療模式使其能夠提供及時可靠的影像結果,這對於有效的臨床決策至關重要。
2024年,美國胰臟癌診斷市場規模達9.386億美元。隨著美國癌症發生率的穩定上升,以及人們認知度的提高和先進醫療服務可近性的不斷提升,對創新診斷技術的需求強勁成長。良好的監管環境、大量的研發投入以及公眾教育活動也促進了市場的成長,使美國成為全球診斷創新的關鍵參與者。
全球胰臟癌診斷市場的領先公司包括通用電氣醫療集團 (GE Healthcare)、賽默飛世爾科技 (Thermo Fisher Scientific)、西門子醫療集團 (Siemens Healthineers)、荷蘭皇家飛利浦 (Koninklijke Philips) 和羅氏製藥 (F. Hoffmann-La Roche)。為了鞏固在胰臟癌診斷市場的領先地位,各大公司正專注於產品創新、人工智慧驅動的診斷工具以及擴大其全球影響力。與醫療保健提供者和研究機構的合作使得新型生物標記和影像技術的共同開發成為可能。各公司也投入精準診斷和微創方法投入巨資,包括液體活體組織切片平台和先進的分子分析技術。下一代診斷試劑盒的監管批准以及成像自動化軟體的整合正在幫助公司獲得競爭優勢。此外,透過數位健康平台擴大可及性以及在新興市場開展策略合作是關鍵的成長舉措。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 胰臟癌發生率不斷上升
- 診斷技術的進步
- 全球醫療保健支出不斷成長
- 提高對早期癌症檢測的認知
- 產業陷阱與挑戰
- 嚴格的監管情景
- 診斷測試成本高昂
- 市場機會
- 人工智慧(AI)在成像技術中的日益融合
- 成長動力
- 成長潛力分析
- 監管格局
- 技術進步
- 當前的技術趨勢
- 新興技術
- 供應鏈分析
- 報銷場景
- 2024年定價分析
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係和合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:按產品,2021 - 2034 年
- 主要趨勢
- 儀器
- 耗材
第6章:市場估計與預測:按測試類型,2021 - 2034 年
- 主要趨勢
- 影像學檢查
- CT掃描
- 磁振造影
- 超音波
- 寵物
- 其他影像學檢查
- 活體組織切片
- 驗血
- 肝功能檢查
- 腫瘤標記
- 其他血液檢查
- 其他測試類型
第7章:市場估計與預測:按癌症類型,2021 - 2034 年
- 主要趨勢
- 外分泌
- 腺癌
- 膠體癌
- 腺鱗癌
- 鱗狀細胞癌
- 內分泌
第8章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 癌症研究機構
- 醫院和診所
- 診斷實驗室
- 診斷影像中心
第9章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- Abbott Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Canon
- Danaher Corporation
- Esaote
- F Hoffmann-La Roche
- GE Healthcare
- Hitachi
- Illumina
- Koninklijke Philips
- Myriad Genetics
- Olympus Corporation
- QIAGEN
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific
The Global Pancreatic Cancer Diagnostic Market was valued at USD 2.5 billion in 2024 and is estimated to grow at a CAGR of 7.2% to reach USD 5 billion by 2034. This growth is largely driven by the increasing global incidence of pancreatic cancer, rising investments in healthcare infrastructure, and ongoing advancements in diagnostic technologies. As awareness about early detection improves and healthcare systems focus on faster diagnosis and improved outcomes, the adoption of advanced diagnostic tools has accelerated. These tools-ranging from molecular diagnostics to high-resolution imaging and biomarker-based assays-play a pivotal role in detecting cancer early, especially in asymptomatic stages when treatment can be most effective.

A wide range of innovative technologies continues to reshape the diagnostic landscape, with AI-integrated imaging, molecular profiling, and liquid biopsy techniques improving accuracy, efficiency, and safety. Additionally, the push toward personalized medicine and patient-centric care is reinforcing the importance of early-stage identification. The expansion of clinical applications, supported by improved workflow efficiencies and minimally invasive procedures, has positioned pancreatic cancer diagnostics as an integral part of modern oncology practices. Increased awareness, combined with the growing demand for timely and precise diagnosis, is creating favorable conditions for market expansion over the next decade.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.5 Billion |
| Forecast Value | $5 Billion |
| CAGR | 7.2% |
In 2024, the consumables segment generated USD 1.4 billion and is forecasted to hit USD 2.8 billion by 2034, with a CAGR of 7%. This segment's strong performance is largely due to the continued use of reagents, assay kits, and testing materials across diagnostic settings. As the demand for liquid biopsy, molecular diagnostics, and immunoassays rises, so does the need for reliable consumables that ensure test consistency, compatibility, and reproducibility. Their role in enabling fast and repeatable testing has made them indispensable in both clinical laboratories and hospital environments.
The exocrine segment held a 92.8% share in 2024, driven by the high prevalence of various exocrine pancreatic cancers, including squamous cell carcinoma, adenosquamous carcinoma, colloid carcinoma, and adenocarcinoma. With most pancreatic cancer cases falling under this category, healthcare providers are prioritizing advanced diagnostic solutions for early detection, accurate staging, and streamlined treatment planning. Emerging technologies such as automated lesion detection, cryo-biopsy innovations, and AI-assisted diagnostics are driving better patient outcomes through faster and more precise results.
The Diagnostic imaging centers segment held a 34.5% share in 2024, attributed to their specialized infrastructure and capability to deliver rapid, high-resolution scans using advanced equipment such as MRI, CT, and endoscopic ultrasound (EUS). These centers continue to play a vital role in facilitating accurate diagnosis, staging, and monitoring of pancreatic cancer. Their integrated workflows and access to cutting-edge modalities allow them to deliver timely, reliable imaging results essential for effective clinical decision-making.
United States Pancreatic Cancer Diagnostic Market reached USD 938.6 million in 2024. The steady rise in cancer incidence across the country, coupled with increasing awareness and access to advanced healthcare, has created strong demand for innovative diagnostic technologies. The favorable regulatory environment, extensive R&D investments, and public education campaigns have also contributed to the growth of the market, positioning the U.S. as a key player in global diagnostics innovation.
Leading companies operating in the Global Pancreatic Cancer Diagnostic Market include GE Healthcare, Thermo Fisher Scientific, Siemens Healthineers, Koninklijke Philips, and F. Hoffmann-La Roche. To strengthen their foothold in the pancreatic cancer diagnostic market, major companies are focusing on product innovation, AI-driven diagnostic tools, and expanding their global presence. Partnerships with healthcare providers and research institutions are enabling co-development of novel biomarkers and imaging techniques. Firms are also investing heavily in precision diagnostics and minimally invasive methods, including liquid biopsy platforms and advanced molecular profiling. Regulatory approvals for next-generation diagnostic kits and integration of software for imaging automation are helping companies gain a competitive advantage. Additionally, expanding access through digital health platforms and strategic collaborations in emerging markets are key growth initiatives.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product trends
- 2.2.3 Test type trends
- 2.2.4 Cancer type trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of pancreatic cancer
- 3.2.1.2 Advancements in diagnostic technologies
- 3.2.1.3 Growing healthcare expenditure globally
- 3.2.1.4 Rising awareness regarding early cancer detection
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory scenarios
- 3.2.2.2 High cost of diagnostic tests
- 3.2.3 Market opportunities
- 3.2.3.1 Rising integration of artificial intelligence (AI) in imaging technology
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Reimbursement scenario
- 3.8 Pricing analysis, 2024
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Instruments
- 5.3 Consumables
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Imaging test
- 6.2.1 CT scan
- 6.2.2 MRI
- 6.2.3 Ultrasound
- 6.2.4 PET
- 6.2.5 Other imaging tests
- 6.3 Biopsy
- 6.4 Blood test
- 6.4.1 Liver function tests
- 6.4.2 Tumor markers
- 6.4.3 Other blood tests
- 6.5 Other test types
Chapter 7 Market Estimates and Forecast, By Cancer Type, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Exocrine
- 7.2.1 Adenocarcinoma
- 7.2.2 Colloid carcinoma
- 7.2.3 Adenosquamous carcinoma
- 7.2.4 Squamous cell carcinoma
- 7.3 Endocrine
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Cancer research institutes
- 8.3 Hospitals and clinics
- 8.4 Diagnostic laboratories
- 8.5 Diagnostic imaging centers
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 Agilent Technologies
- 10.3 Becton, Dickinson and Company
- 10.4 Boston Scientific Corporation
- 10.5 Canon
- 10.6 Danaher Corporation
- 10.7 Esaote
- 10.8 F Hoffmann-La Roche
- 10.9 GE Healthcare
- 10.10 Hitachi
- 10.11 Illumina
- 10.12 Koninklijke Philips
- 10.13 Myriad Genetics
- 10.14 Olympus Corporation
- 10.15 QIAGEN
- 10.16 Siemens Healthineers
- 10.17 Sysmex Corporation
- 10.18 Thermo Fisher Scientific










