 |
市場調查報告書
商品編碼
1801898
HIV 診斷市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測HIV Diagnostics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
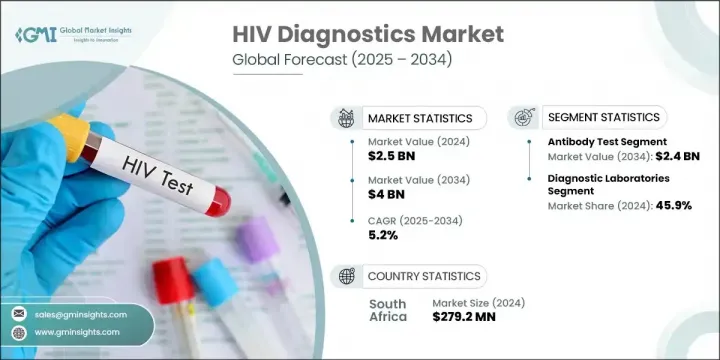
2024年,全球愛滋病毒診斷市場規模達25億美元,預估年複合成長率將達5.2%,2034年將達40億美元。這一成長趨勢得益於中低收入地區愛滋病毒感染率的上升,以及對更可靠、更便利的診斷方法的需求。隨著全球愛滋病毒檢測意識的提升,公共和私人醫療體係都在擴大採用先進的檢測解決方案。即時診斷因其快速、便捷且適用於基礎設施匱乏的地區,正日益受到青睞。
用於識別愛滋病毒感染的診斷技術在早期介入、患者監測和確定合適的治療方案方面發揮核心作用。全球各種衛生工作和資助計畫持續推動對便利的愛滋病毒檢測解決方案的需求。隨著醫療保健可近性和健康素養的提高,愛滋病毒檢測試劑盒的採用率持續上升,尤其是在醫療資源匱乏的社區。技術創新以及更快速、更經濟的檢測解決方案進一步支持了其在公共衛生計劃和臨床環境中的廣泛應用。政策支持、公眾意識的提升以及診斷技術的快速進步,這些因素共同重塑了全球愛滋病毒診斷格局。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 25億美元 |
| 預測值 | 40億美元 |
| 複合年成長率 | 5.2% |
抗體檢測細分市場在2024年引領市場,並憑藉其成本效益、簡單易行和快速出結果的優勢佔據最大佔有率。抗體檢測在自測試劑盒中的日益普及也促進了其廣泛應用,尤其是在資源匱乏的地區。這些檢測是大規模篩檢的理想選擇,因為它們易於取得且易於操作,無需複雜的醫療環境。隨著越來越多的人選擇注重隱私的自測方法,抗體檢測的重要性日益提升,尤其是在那些有醫療污名或就診管道受限等問題的地區。
2024年,診斷實驗室市場佔有45.9%的佔有率。這些設施仍然是HIV檢測的首選,因為它們能夠有效地管理大量檢測。隨著HIV感染率的上升,對準確、高通量檢測的需求也不斷增加。實驗室利用先進的儀器和自動化系統來提供可靠的結果,這使得它們對於支援公共衛生計畫和大規模檢測活動至關重要。
2024年,中東和非洲愛滋病毒診斷市場佔據30.1%的市場。該地區不斷上升的感染率持續刺激著對更有效、更便利的診斷工具的需求。隨著人們的意識和資源投入到控制愛滋病毒傳播方面,對可擴展且精準的檢測解決方案的需求將繼續推動市場發展。
HIV 診斷市場的領先公司包括 Bioneer、Hologic、Genlantis Diagnostics、Qiagen、ChemBio Diagnostics、OraSure Technologies、Becton, Dickinson and Company (BD)、F. Hoffmann-La Roche、Abbott Laboratories、Biomerieux 和 Cepheid。 HIV 診斷領域的主要參與者正在透過擴展檢測組合、增強即時診斷平台以及投資研發來鞏固其市場地位。許多公司正在推出下一代診斷試劑盒,這些試劑盒注重速度、便攜性和準確性,專為分散的醫療保健環境量身定做。
與醫療機構、非政府組織和政府的合作有助於企業獲得大規模供應契約,而透過本地合作夥伴關係進行區域擴張則能增強分銷能力。為了保持競爭力,一些公司正在將數位工具與檢測試劑盒整合,以實現即時資料追蹤和遠端監控。其他公司則專注於監管部門的核准和認證,以加速進入服務匱乏、愛滋病毒感染率不斷上升的地區市場。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 中低收入國家愛滋病/愛滋病毒感染率上升
- 政府加大愛滋病毒宣導力度
- HIV即時診斷技術的發展
- 美國有利的監管環境
- 產業陷阱與挑戰
- 低度開發市場滲透率低
- 社會恥辱和歧視
- 市場機會
- 持續的技術進步
- 與數位健康平台整合
- 成長動力
- 成長潛力分析
- 監管格局
- 美國
- 歐洲
- 技術進步
- 2024年定價分析
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 戰略展望矩陣
- 戰略儀表板
- 關鍵進展
- 併購
- 夥伴關係和合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:按測試類型,2021 - 2034 年
- 主要趨勢
- 抗體檢測
- 病毒量測試
- CD4偵測
第6章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 診斷實驗室
- 醫院和診所
- 家庭設定
- 其他最終用途
第7章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第8章:公司簡介
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- Biomerieux
- Bioneer
- Cepheid
- ChemBio Diagnostics
- F. Hoffmann-La Roche
- Genlantis Diagnostics
- Hologic
- OraSure Technologies
- Qiagen
The Global HIV Diagnostics Market was valued at USD 2.5 billion in 2024 and is estimated to grow at a CAGR of 5.2% to reach USD 4 billion by 2034. This upward trend is supported by rising HIV incidence in lower- and middle-income regions, combined with the demand for more reliable and accessible diagnostic methods. As awareness surrounding HIV testing grows through global campaigns, both public and private healthcare systems are increasingly adopting advanced testing solutions. Point-of-care diagnostics are gaining momentum due to their speed, convenience, and suitability in regions with limited infrastructure.

Diagnostic technologies used to identify HIV infections play a central role in early intervention, patient monitoring, and determining suitable treatment paths. Various global health efforts and funding initiatives continue to create demand for accessible HIV testing solutions. As access to healthcare and health literacy improve, the adoption of HIV testing kits, especially in underserved communities, continues to rise. Technological innovation and faster, cost-efficient testing solutions are further supporting widespread adoption across both public health initiatives and clinical settings. The combination of policy support, increasing public awareness, and rapid diagnostic advancements continues to reshape the HIV diagnostics landscape globally.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.5 Billion |
| Forecast Value | $4 Billion |
| CAGR | 5.2% |
The antibody tests segment led the market in 2024 and held the largest share due to their cost-effectiveness, simplicity, and ability to deliver quick results. Their growing use in self-testing kits has also contributed to widespread adoption, particularly in low-resource environments. These tests are ideal for mass screening as they are both accessible and easy to administer without requiring complex medical settings. Their relevance continues to grow as more individuals opt for privacy-driven self-testing methods, especially in regions where healthcare stigma or limited access to clinics is a concern.
The diagnostic laboratories segment held 45.9% share in 2024. These facilities remain the preferred choice for HIV testing because they are equipped to manage a large volume of tests efficiently. With growing HIV prevalence, the need for accurate and high-throughput testing is expanding. Laboratories utilize sophisticated instruments and automated systems to deliver reliable results, making them essential for supporting public health programs and large-scale testing campaigns.
Middle East and Africa HIV Diagnostics Market held 30.1% share in 2024. Rising infection rates in this region continue to fuel demand for more effective and accessible diagnostic tools. As more awareness and resources are directed toward managing the spread of HIV, the need for scalable and accurate testing solutions continues to drive the market forward.
Leading companies in the HIV Diagnostics Market include Bioneer, Hologic, Genlantis Diagnostics, Qiagen, ChemBio Diagnostics, OraSure Technologies, Becton, Dickinson and Company (BD), F. Hoffmann-La Roche, Abbott Laboratories, Biomerieux, and Cepheid. Major players in the HIV diagnostics sector are reinforcing their market presence by expanding testing portfolios, enhancing point-of-care platforms, and investing in R&D. Many are launching next-generation diagnostic kits that prioritize speed, portability, and accuracy, tailored for decentralized healthcare settings.
Collaborations with healthcare agencies, NGOs, and governments help companies secure large-scale supply contracts, while regional expansion through local partnerships strengthens distribution. To remain competitive, several firms are integrating digital tools with testing kits, enabling real-time data tracking and remote monitoring. Others focus on regulatory approvals and certifications to accelerate market entry in underserved regions with rising HIV incidence.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Test type
- 2.2.3 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising AIDS/HIV prevalence in low and middle-income countries
- 3.2.1.2 Increasing government initiatives for HIV awareness
- 3.2.1.3 Point-of-care (POC) HIV diagnostics development
- 3.2.1.4 Favorable regulatory landscape in the U.S.
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Low degree of penetration in underdeveloped market
- 3.2.2.2 Social stigma and discrimination
- 3.2.3 Market opportunities
- 3.2.3.1 Ongoing technology advancement
- 3.2.3.2 Integration with digital health platforms
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.5 Technological advancements
- 3.6 Pricing analysis, 2024
- 3.7 Future market trends
- 3.8 Gap analysis
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategic outlook matrix
- 4.7 Strategic dashboard
- 4.8 Key developments
- 4.8.1 Mergers and acquisitions
- 4.8.2 Partnerships and collaborations
- 4.8.3 New product launches
- 4.8.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Antibody test
- 5.3 Viral load test
- 5.4 CD4 test
Chapter 6 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Diagnostic laboratories
- 6.3 Hospitals and clinics
- 6.4 Home settings
- 6.5 Other end use
Chapter 7 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 North America
- 7.2.1 U.S.
- 7.2.2 Canada
- 7.3 Europe
- 7.3.1 Germany
- 7.3.2 UK
- 7.3.3 France
- 7.3.4 Spain
- 7.3.5 Italy
- 7.3.6 Netherlands
- 7.4 Asia Pacific
- 7.4.1 China
- 7.4.2 India
- 7.4.3 Japan
- 7.4.4 Australia
- 7.4.5 South Korea
- 7.5 Latin America
- 7.5.1 Brazil
- 7.5.2 Mexico
- 7.5.3 Argentina
- 7.6 Middle East and Africa
- 7.6.1 Saudi Arabia
- 7.6.2 South Africa
- 7.6.3 UAE
Chapter 8 Company Profiles
- 8.1 Abbott Laboratories
- 8.2 Becton, Dickinson and Company (BD)
- 8.3 Biomerieux
- 8.4 Bioneer
- 8.5 Cepheid
- 8.6 ChemBio Diagnostics
- 8.7 F. Hoffmann-La Roche
- 8.8 Genlantis Diagnostics
- 8.9 Hologic
- 8.10 OraSure Technologies
- 8.11 Qiagen










