 |
市場調查報告書
商品編碼
1797862
填充飾面製造市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Fill Finish Manufacturing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球灌裝封口製造市場規模達136億美元,預計2034年將以9.3%的複合年成長率成長,達到328億美元。市場成長的動力源於製造技術的不斷發展、對無菌給藥方式的需求不斷成長以及生物製藥開發的顯著擴張。灌裝封口製造在確保腸外給藥產品的無菌性、精密性和安全包裝方面發揮著至關重要的作用。它涉及將疫苗、生物製劑和其他注射劑型無菌填充到預充式注射器、西林瓶、安瓿瓶和卡式瓶等包裝中。
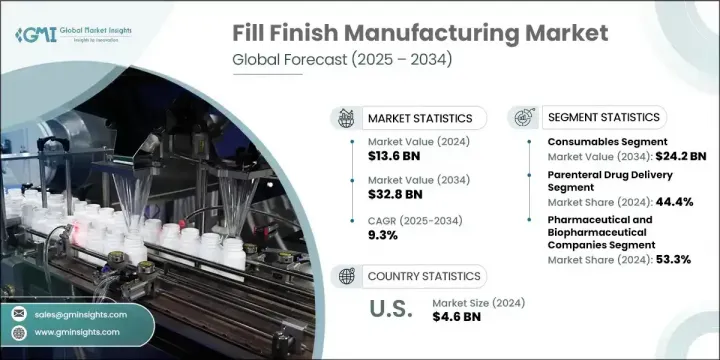
預灌封注射器日益普及,正在重塑市場格局,這得益於其能夠最大程度地減少污染和劑量錯誤,尤其是在複雜的生物製劑領域。製藥公司正在加大對高效灌裝系統、先進無菌包裝和數位化品質控制工具的投資,以滿足日益成長的需求並遵守不斷變化的監管框架。注射用生物製劑的核准以及即用型 (RTU) 容器系統的興起,進一步增強了精密設計的填充解決方案的需求。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 136億美元 |
| 預測值 | 328億美元 |
| 複合年成長率 | 9.3% |
2024年,耗材市場規模達102億美元,預計2034年將達242億美元。卡式瓶、西林瓶和預充式注射器等耗材廣泛應用於注射劑的配製和輸送。這些耗材對於維持無菌、精準劑量和降低污染風險至關重要。其高消耗量和一次性使用的特性使其成為現代製藥生產環境中灌裝製程不可或缺的組成部分。在生物製劑和疫苗生產中,這些耗材的應用尤其廣泛,因為這些生產環節對絕對精確度和污染控制至關重要。
2024年,製藥和生物製藥公司佔了53.3%的市場。由於其在無菌注射劑的商業化生產和包裝領域擁有大量業務,該領域繼續保持領先地位。這些公司依靠先進的填充線、封閉式隔離器和智慧機器人技術,確保其產品線的精準性、無菌性和合規性。他們廣泛的藥物開發管線,包括個人化療法和單株抗體,推動了對高度專業化的填充設備和耗材的需求,這些設備和耗材能夠處理複雜的產品特性和客製化規格。
2024年,北美灌裝包裝製造市場佔37%的市佔率。該地區高度發達的製藥基礎設施和強大的合約製造組織幫助其持續成長。其監管環境支持創新,同時強調安全和品質。北美製造商正在快速整合自動化技術和無菌加工能力,以滿足不斷成長的產量並適應更新的生物製劑配方。先進的包裝系統和無菌耗材的供應進一步鞏固了該地區在全球產量中的主導地位。
灌裝封口製造市場的知名公司包括 Gerresheimer、Corning Incorporated、AST、SGD Pharma、SCHOTT Pharma、Stevanato Group、IMA Industria Macchine Automatiche、Steriline、Becton、Dickinson and Company、Bausch+Strobelific、Nipro、Groninger、Kishore Group、Bquinsilent 和 Maunar。這些公司繼續在從設備到耗材的各個領域佔據強勢地位。活躍於灌裝封口製造市場的公司正在積極投資下一代自動化、機器人和模組化生產線,以提高營運靈活性和增加產量。與生物製藥開發商的策略合作幫助這些參與者共同開發容器形式並縮短產品開發時間。對法規遵從性和品質保證的高度關注推動了對無塵室技術和無菌隔離器的持續投資。主要參與者也在擴大其全球製造足跡,以滿足特定區域的需求並縮短交貨時間。為了滿足個人化藥品和小批量生產日益成長的需求,一些公司正在創新多規格機器和軟性填充生產線。此外,公司正在加強研發力度,開發即用型包裝組件,以降低填充工序的複雜性,同時提高製程可靠性。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 供應商格局
- 每個階段的增值
- 影響價值鏈的因素
- 產業衝擊力
- 成長動力
- 填充飾面製造程序的技術進步日新月異
- 生物製藥產業的成長
- 預充式注射器在腸外給藥的應用日益增多
- 產業陷阱與挑戰
- 隔離器/限制進入屏障系統(RABS)成本高昂
- 嚴格的監管問題
- 市場機會
- 新興市場的擴張
- 即用型(RTU)容器的採用率不斷提高
- 成長動力
- 成長潛力分析
- 技術和創新格局
- 當前的技術趨勢
- 新興技術
- 監管格局
- 未來市場趨勢
- 定價分析
- 按產品
- 差距分析
- 供應鏈和分銷分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 按地區
- 北美洲
- 歐洲
- 亞太地區
- 按地區
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係和合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:按產品,2021 - 2034 年
- 主要趨勢
- 耗材
- 預充式注射器(PFS)
- 玻璃纖維增強塑膠
- 塑膠PFS
- 小瓶
- 玻璃小瓶
- 塑膠小瓶
- 墨水匣
- 其他耗材
- 預充式注射器(PFS)
- 儀器
- 類型
- 整合系統
- 獨立系統
- 手術
- 自動化機器
- 半自動和手動機器
- 類型
第6章:市場估計與預測:按應用,2021 - 2034 年
- 主要趨勢
- 腸外給藥
- 生物製劑製造
- 個人化醫療
- 其他應用
第7章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 製藥和生物製藥公司
- 合約製造組織
- 其他最終用途
第8章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- 全球參與者
- Becton, Dickinson and Company
- Bausch+Strobel
- Corning
- Gerresheimer
- IMA Industria Macchine Automatiche
- Nipro Corporation
- SCHOTT Pharma
- SGD Pharma
- Stevanato Group
- 區域參與者
- Groninger
- Maquinaria Industrial Dara
- Steriline
- 新興企業
- AST
- Borosil Scientific
- Kishore Group
The Global Fill Finish Manufacturing Market was valued at USD 13.6 billion in 2024 and is estimated to grow at a CAGR of 9.3% to reach USD 32.8 billion by 2034. Market growth is being powered by the continued evolution of manufacturing technologies, increasing demand for sterile drug delivery formats, and a significant expansion in biopharmaceutical development. Fill finish manufacturing plays a vital role in ensuring the sterility, precision, and safe packaging of parenteral drug products. It involves the aseptic filling of vaccines, biologics, and other injectable formulations into formats like prefilled syringes, vials, ampoules, and cartridges.

The growing popularity of prefilled syringes is reshaping this market, driven by their ability to minimize contamination and dosage errors, particularly in the case of complex biologics. Pharmaceutical firms are increasing investment in high-efficiency filling systems, advanced sterile packaging, and digital quality control tools to meet rising demand and comply with evolving regulatory frameworks. The combination of injectable biologic approvals and the rise of Ready-To-Use (RTU) container systems is further enhancing the demand for precision-engineered fill finish solutions.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $13.6 Billion |
| Forecast Value | $32.8 Billion |
| CAGR | 9.3% |
In 2024, the consumables segment generated USD 10.2 billion and is projected to hit USD 24.2 billion by 2034. Consumables such as cartridges, vials, and prefilled syringes are used extensively in the formulation and delivery of injectable medications. These items are indispensable in preserving sterility, delivering accurate doses, and reducing the risk of contamination. Their high-volume consumption and single-use nature make them an integral component of the fill finish process in modern pharmaceutical manufacturing environments. Their adoption is especially high in biologics and vaccine production, where absolute precision and contamination control are paramount.
The pharmaceutical and biopharmaceutical companies segment held a 53.3% share in 2024. This segment continues to lead due to its substantial involvement in the commercial-scale production and packaging of sterile injectable drugs. These companies rely on advanced filling lines, closed-system isolators, and intelligent robotics to ensure precision, sterility, and regulatory compliance across their product pipelines. Their expansive drug development pipelines, including personalized therapies and monoclonal antibodies, are driving the need for highly specialized fill finish equipment and consumables that can handle complex product characteristics and customized formats.
North America Fill Finish Manufacturing Market held a 37% share in 2024. The region's highly developed pharmaceutical infrastructure and the strong presence of contract manufacturing organizations contribute to sustained growth. Its regulatory landscape supports innovation while emphasizing safety and quality. North American manufacturers are rapidly integrating automated technologies and aseptic processing capabilities to meet rising production volumes and adapt to newer biologic formulations. The availability of advanced packaging systems and sterile consumables further supports the region's dominance in global production output.
Prominent companies operating in the Fill Finish Manufacturing Market include Gerresheimer, Corning Incorporated, AST, SGD Pharma, SCHOTT Pharma, Stevanato Group, I.M.A. Industria Macchine Automatiche, Steriline, Becton, Dickinson and Company, Bausch+Strobel, Nipro, Groninger, Kishore Group, Borosil Scientific, and Maquinaria Industrial Dara. These firms continue to hold a strong foothold across various segments of the market, from equipment to consumables. Companies active in the fill finish manufacturing market are investing aggressively in next-gen automation, robotics, and modular production lines to improve operational flexibility and increase throughput. Strategic collaborations with biopharmaceutical developers help these players co-develop container formats and reduce product development timelines. A strong focus on regulatory compliance and quality assurance drives ongoing investments in cleanroom technology and aseptic isolators. Major players are also expanding their global manufacturing footprint to serve region-specific demand and reduce lead times. To cater to the rising demand for personalized drugs and small-batch production, several firms are innovating with multi-format machines and flexible filling lines. In addition, enhanced R&D efforts are being channeled into developing RTU packaging components that reduce fill finish complexities while improving process reliability.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Product
- 2.2.2 Application
- 2.2.3 End use
- 2.2.4 Regional
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising technological advancements in fill finish manufacturing processes
- 3.2.1.2 Growth of the biopharmaceutical industry
- 3.2.1.3 Rising adoption of prefilled syringes for parenteral dosage forms
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost associated with isolators/restricted access barrier systems (RABS)
- 3.2.2.2 Stringent regulatory issues
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion in emerging markets
- 3.2.3.2 Increasing adoption of ready-to-use (RTU) containers
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Technology and innovation landscape
- 3.4.1 Current technological trends
- 3.4.2 Emerging technologies
- 3.5 Regulatory landscape
- 3.5.1 North America
- 3.5.2 Europe
- 3.5.3 Asia Pacific
- 3.5.4 Latin America
- 3.5.5 Middle East and Africa
- 3.6 Future market trends
- 3.7 Pricing analysis
- 3.7.1 By product
- 3.8 Gap analysis
- 3.9 Supply chain and distribution analysis
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 By region
- 4.2.1.1 North America
- 4.2.1.2 Europe
- 4.2.1.3 Asia Pacific
- 4.2.1 By region
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Consumables
- 5.2.1 Prefilled syringes (PFS)
- 5.2.1.1 Glass PFS
- 5.2.1.2 Plastic PFS
- 5.2.2 Vials
- 5.2.2.1 Glass vials
- 5.2.2.2 Plastic vials
- 5.2.3 Cartridges
- 5.2.4 Other consumables
- 5.2.1 Prefilled syringes (PFS)
- 5.3 Instruments
- 5.3.1 Type
- 5.3.1.1 Integrated systems
- 5.3.1.2 Standalone systems
- 5.3.2 Operation
- 5.3.2.1 Automated machines
- 5.3.2.2 Semi-automated and manual machines
- 5.3.1 Type
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Parenteral drug delivery
- 6.3 Biologics manufacturing
- 6.4 Personalized medicines
- 6.5 Other applications
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical and biopharmaceutical companies
- 7.3 Contract manufacturing organizations
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Global players
- 9.1.1 Becton, Dickinson and Company
- 9.1.2 Bausch+Strobel
- 9.1.3 Corning
- 9.1.4 Gerresheimer
- 9.1.5 I.M.A. Industria Macchine Automatiche
- 9.1.6 Nipro Corporation
- 9.1.7 SCHOTT Pharma
- 9.1.8 SGD Pharma
- 9.1.9 Stevanato Group
- 9.2 Regional players
- 9.2.1 Groninger
- 9.2.2 Maquinaria Industrial Dara
- 9.2.3 Steriline
- 9.3 Emerging players
- 9.3.1 AST
- 9.3.2 Borosil Scientific
- 9.3.3 Kishore Group












