 |
市場調查報告書
商品編碼
1797754
藥品阻隔包裝材料市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Barrier Packaging Materials for Pharmaceuticals Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
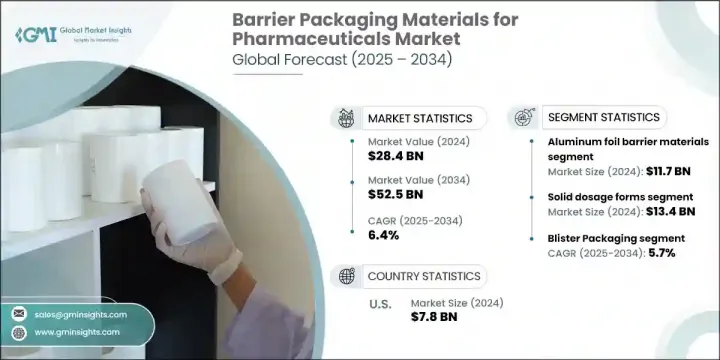
2024 年全球藥品阻隔包裝材料市場價值為 284 億美元,預計到 2034 年將以 6.4% 的複合年成長率成長,達到 525 億美元。 2024 年全球藥品阻隔包裝材料市場價值為 284 億美元,預計到 2034 年將以 6.4% 的複合年成長率成長,達到 525 億美元。對保護藥品免受濕氣、氧氣、光線和微生物污染的包裝的需求不斷成長,推動了高阻隔材料的應用。這些解決方案對於維持現代藥物製劑的化學穩定性和治療價值至關重要。隨著生物製劑、個人化醫療和複雜藥物成分的興起,包裝要求變得更加嚴格,促使製造商投資於提供卓越保護的先進材料。多層層壓板、PVDC 塗層和鋁基薄膜等阻隔技術因其與多種劑型相容且符合全球監管標準而獲得廣泛認可。
製造商越來越重視開發可回收、無溶劑的阻隔材料,以滿足永續發展需求和不斷發展的醫藥需求。精準治療的趨勢增加了對耐熱性和防潮性更強的新型材料的需求。為了應對這些挑戰,製造商正在探索包括奈米塗層和混合結構在內的包裝創新技術,以延長保存期限並確保產品在全球分銷過程中的完整性。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 284億美元 |
| 預測值 | 525億美元 |
| 複合年成長率 | 6.4% |
2024年,鋁箔阻隔材料市場規模達117億美元,憑藉其在保護吸濕性藥物、藥片和膠囊等敏感產品方面的卓越性能,維持了強勁的市場地位。冷成型和帶狀包裝箔尤其適用於對濕度敏感且穩定性至關重要的應用。
固體劑型市場在2024年創造了134億美元的市場規模,預計到2034年將以5.9%的複合年成長率成長。這些產品主要為片劑和膠囊,需要具有強大阻隔性能的包裝,以適應各種儲存和運輸環境。高性能薄膜和鋁箔已成為確保產品壽命和維持療效的標準。
2024年,美國醫藥阻隔包裝材料市場規模達78億美元,佔82%的市佔率。由於製藥業的擴張、學名藥產量的提高以及先進藥物輸送技術的創新,美國市場將繼續保持成長勢頭。隨著對生物製劑、生物相似藥和溶劑型活性成分等複雜製劑的保護需求不斷成長,企業正在加速向更具彈性、響應速度更快的包裝解決方案轉型。
塑造全球醫藥阻隔包裝材料市場的關鍵公司包括 Winpak Ltd.、Amcor plc、Sealed Air Corporation、Constantia flexibles 和 Berry Global Inc.。為了維持並擴大市場佔有率,醫藥阻隔包裝領域的領先公司正在部署一系列策略方針。他們大力投資研發,以開發符合環境和藥品監管標準的高阻隔、可回收和單一材料薄膜。與藥廠建立策略夥伴關係,幫助他們跟上不斷變化的配方需求。許多公司也透過採用自動化和智慧製造來提高生產能力,以確保一致性和可擴展性。透過併購和區域製造中心擴大全球影響力,這些公司能夠滿足新興市場日益成長的需求。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 供應商概況
- 利潤率
- 每個階段的增值
- 影響價值鏈的因素
- 中斷
- 產業衝擊力
- 成長動力
- 提高藥物穩定性的要求。
- 生物製劑和特種藥物的成長
- 監理合規要求
- 產業陷阱與挑戰
- 材料和加工成本高
- 複雜的法規核准流程
- 環境與永續發展壓力
- 市場機會
- 成長動力
- 成長潛力分析
- 監管格局
- 北美洲
- 歐洲
- 亞太地區
- 拉丁美洲
- 中東和非洲
- 波特的分析
- PESTEL分析
- 價格趨勢
- 按地區
- 未來市場趨勢
- 技術和創新格局
- 當前的技術趨勢
- 新興技術
- 專利格局
- 貿易統計(HS編碼)(註:僅提供重點國家的貿易統計資料)
- 主要進口國
- 主要出口國
- 永續性和環境方面
- 永續實踐
- 減少廢棄物的策略
- 生產中的能源效率
- 環保舉措
- 碳足跡考量
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 按地區
- 北美洲
- 歐洲
- 亞太地區
- 拉丁美洲
- MEA
- 按地區
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:按材料類型,2021 - 2034 年
- 主要趨勢
- 鋁箔阻隔材料
- 純鋁箔
- 標準厚等級
- 高性能合金
- 特殊表面處理
- 層壓鋁結構
- 鋁聚合物層壓板
- 鋁紙複合材料
- 多層鋁系統
- 純鋁箔
- 聚合物薄膜阻隔材料
- 塗層鋁解決方案
- 聚合物塗層鋁
- 功能塗層應用
- 效能增強技術
- PVDC(聚偏二氯乙烯)系統
- 塗層應用
- 薄膜配方
- EVOH(乙烯乙烯醇)阻隔材料
- 阻隔膜應用
- 層壓系統
- PET(聚對苯二甲酸乙二醇酯)薄膜
- 標準寵物圍欄
- 強化屏障寵物
- 特殊配方
- 其他聚合物屏障
- PE(聚乙烯)系統
- PP(聚丙烯)屏障
- 特種聚合物解決方案
- 塗層鋁解決方案
- 多層阻隔系統
- 層壓結構
- 黏合劑基底層壓板
- 擠壓塗層系統
- 熱黏合結構
- 共擠屏障
- 多層共擠
- 連接層技術
- 效能最佳化
- 層壓結構
- 先進的阻隔技術
- 奈米科技增強屏障
- 等離子沉積塗層
- 原子層沉積系統
第6章:市場估計與預測:按應用,2021 - 2034 年
- 主要趨勢
- 固體劑型
- 片劑和囊片
- 速釋製劑
- 緩釋系統
- 專業平板電腦應用
- 膠囊
- 硬明膠膠囊
- 軟膠囊
- 腸溶膠囊
- 粉末和顆粒
- 散裝粉末包裝
- 單劑量小袋
- 重組產品
- 片劑和囊片
- 液體劑型
- 口服液
- 糖漿和混懸液
- 溶液和乳液
- 兒科製劑
- 注射溶液
- 小瓶和安瓿瓶
- 預充式注射器
- 靜脈輸液袋和容器
- 外用製劑
- 乳膏和藥膏
- 凝膠和乳液
- 透皮系統
- 口服液
- 生物製劑和生物相似藥
- 蛋白質療法
- 單株抗體
- 疫苗
- 酵素療法
- 細胞和基因療法
- 活細胞產品
- 基因治療載體
- 再生醫學
- 蛋白質療法
- 特種藥品
- 管制物質
- 孤兒藥
- 個人化醫療
- 臨床試驗材料
第7章:市場估計與預測:依包裝形式,2021 - 2034 年
- 主要趨勢
- 吸塑包裝
- 熱成型泡殼
- PVC泡殼系統
- 聚氯乙烯塗層泡殼
- 鋁鋁泡殼
- 冷成型泡殼
- 鋁基系統
- 高阻隔性能
- 專業應用
- 特殊泡殼形式
- 兒童安全泡殼
- 適合老年人的設計
- 單位劑量系統
- 熱成型泡殼
- 軟包裝
- 小袋和小袋
- 單劑量袋裝
- 多劑量包裝
- 棒狀包裝
- 袋子和包裝
- 散裝包裝解決方案
- 保護性包裝
- 中間包裝
- 小袋和小袋
- 硬質包裝
- 瓶子和容器
- 塑膠瓶系統
- 玻璃容器應用
- 增強屏障的封閉
- 小瓶和安瓿瓶
- 玻璃瓶系統
- 塑膠瓶應用
- 專用封蓋
- 瓶子和容器
- 特殊包裝形式
- 預充式注射器
- 墨水匣系統
- 吸入裝置
- 透皮貼劑
第8章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 歐洲其他地區
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 亞太其他地區
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 拉丁美洲其他地區
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
- 中東和非洲其他地區
第9章:公司簡介
- Amcor Plc
- Constantia Flexibles Group GmbH
- Klockner Pentaplast Group
- Tekni-Plex, Inc
- Uflex Limited
- Mondi Group
- Sealed Air Corporation
- Winpak Ltd
- Huhtamaki Oyj
- Berry Global Inc
- CCL Industries Inc
- Schott AG
- Gerresheimer AG
- West Pharmaceutical Services, Inc
- Sonoco Products Company
The Global Barrier Packaging Materials for Pharmaceuticals Market was valued at USD 28.4 billion in 2024 and is estimated to grow at a CAGR of 6.4% to reach USD 52.5 billion by 2034. The Global Barrier Packaging Materials for Pharmaceuticals Market was valued at USD 28.4 billion in 2024 and is estimated to grow at a CAGR of 6.4% to reach USD 52.5 billion by 2034. The increasing demand for packaging that protects pharmaceutical products from moisture, oxygen, light, and microbial contamination is driving the adoption of high-barrier materials. These solutions are essential for preserving the chemical stability and therapeutic value of modern drug formulations. With the rise in biologics, personalized medicine, and complex drug compositions, packaging requirements have become more stringent, leading manufacturers to invest in advanced materials that offer superior protection. Barrier technologies such as multilayer laminates, PVDC coatings, and aluminum-based films have gained widespread acceptance due to their compatibility with a wide range of dosage forms and adherence to global regulatory standards.

Manufacturers are placing growing emphasis on developing recyclable, solvent-free barrier materials that comply with sustainability mandates and evolving pharmaceutical requirements. The trend toward precision therapies has increased demand for novel materials with improved thermal resistance and moisture control. Packaging innovations, including nanocoatings and hybrid structures, are being explored to meet these challenges while extending shelf life and ensuring product integrity during global distribution.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $28.4 Billion |
| Forecast Value | $52.5 Billion |
| CAGR | 6.4% |
The aluminum foil barrier materials segment accounted for USD 11.7 billion in 2024, maintaining a strong foothold due to their exceptional performance in protecting sensitive products like hygroscopic drugs, tablets, and capsules. Cold-form and strip-pack foils are particularly preferred in moisture-sensitive applications where stability is critical.
The solid dosage segment generated USD 13.4 billion in 2024 and is expected to grow at a CAGR of 5.9% through 2034. These products, primarily tablets and capsules, require packaging with robust barrier properties to withstand diverse storage and transit environments. High-performance films and aluminum foils have become standard in ensuring product longevity and maintaining therapeutic efficacy.
U.S. Barrier Packaging Materials for Pharmaceuticals Market generated USD 7.8 billion in 2024, capturing 82% share. Growth in the country continues due to expanding pharmaceutical manufacturing, increased generic drug output, and innovation in advanced drug delivery technologies. As the demand rises for protecting complex formulations such as biologics, biosimilars, and solvent-based active ingredients, the shift to more resilient and responsive packaging solutions is accelerating.
Key companies shaping the Global Barrier Packaging Materials for Pharmaceuticals Market include Winpak Ltd., Amcor plc, Sealed Air Corporation, Constantia Flexibles, and Berry Global Inc. To maintain and grow their market presence, leading companies in the pharmaceutical barrier packaging sector are deploying a range of strategic approaches. They are heavily investing in R&D to develop high-barrier, recyclable, and mono-material films that meet both environmental and pharmaceutical regulatory standards. Strategic partnerships with drug manufacturers help them stay aligned with evolving formulation needs. Many firms are also enhancing production capabilities by adopting automation and smart manufacturing to ensure consistency and scalability. Expanding their global reach through mergers, acquisitions, and regional manufacturing hubs allows these players to address rising demand in emerging markets.
Table of Contents
Chapter 1 Methodology & Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Material type
- 2.2.3 Application
- 2.2.4 Packaging format
- 2.3 TAM ANALYSIS, 2025-2034
- 2.4 CXO PERSPECTIVES: strategic imperatives
- 2.4.1 Executive decision points
- 2.4.2 Critical success factors
- 2.5 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier Landscape
- 3.1.2 Profit Margin
- 3.1.3 Value addition at each stage
- 3.1.4 Factor affecting the value chain
- 3.1.5 Disruptions
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing drug stability requirements.
- 3.2.1.2 Biologics and specialty drug growth
- 3.2.1.3 Regulatory compliance mandates
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High material and processing costs
- 3.2.2.2 Complex regulatory approval processes
- 3.2.2.3 Environmental and sustainability pressures
- 3.2.3 Market opportunities
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East & Africa
- 3.5 Porter's analysis
- 3.6 PESTEL analysis
- 3.6.1 Technology and Innovation Landscape
- 3.6.2 Current technological trends
- 3.6.3 Emerging technologies
- 3.7 Price trends
- 3.7.1 By region
- 3.8 Future market trends
- 3.9 Technology and innovation landscape
- 3.9.1 Current technological trends
- 3.9.2 Emerging technologies
- 3.10 Patent landscape
- 3.11 Trade statistics (HS code) (Note: the trade statistics will be provided for key countries only)
- 3.11.1 Major importing countries
- 3.11.2 Major exporting countries
- 3.12 Sustainability and environmental aspects
- 3.12.1 Sustainable practices
- 3.12.2 Waste reduction strategies
- 3.12.3 Energy efficiency in production
- 3.12.4 Eco-friendly initiatives
- 3.13 Carbon footprint considerations
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 By region
- 4.2.1.1 North America
- 4.2.1.2 Europe
- 4.2.1.3 Asia Pacific
- 4.2.1.4 LATAM
- 4.2.1.5 MEA
- 4.2.1 By region
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Material Type, 2021 - 2034 (USD Billion) (Kilo Tons)
- 5.1 Key trends
- 5.2 Aluminum foil barrier materials
- 5.2.1 Pure aluminum foil
- 5.2.1.1 Standard thickness grades
- 5.2.1.2 High-performance alloys
- 5.2.1.3 Specialized surface treatments
- 5.2.2 Laminated aluminum structures
- 5.2.2.1 Aluminum-polymer laminates
- 5.2.2.2 Aluminum-paper composites
- 5.2.2.3 Multi-layer aluminum systems
- 5.2.1 Pure aluminum foil
- 5.3 Polymer film barrier materials
- 5.3.1 Coated aluminum solutions
- 5.3.1.1 Polymer-coated aluminum
- 5.3.1.2 Functional coating applications
- 5.3.1.3 Performance enhancement technologies
- 5.3.2 PVDC (polyvinylidene chloride) systems
- 5.3.2.1 Coating applications
- 5.3.2.2 Film formulations
- 5.3.3 EVOH (ethylene vinyl alcohol) barriers
- 5.3.3.1 Barrier film applications
- 5.3.3.2 Lamination systems
- 5.3.4 PET (polyethylene terephthalate) films
- 5.3.4.1 Standard pet barriers
- 5.3.4.2 Enhanced barrier pet
- 5.3.4.3 Specialty formulations
- 5.3.5 Other polymer barriers
- 5.3.5.1 PE (polyethylene) systems
- 5.3.5.2 PP (polypropylene) barriers
- 5.3.5.3 Specialty polymer solutions
- 5.3.1 Coated aluminum solutions
- 5.4 Multi-layer barrier systems
- 5.4.1 Laminated structures
- 5.4.1.1 Adhesive-based laminates
- 5.4.1.2 Extrusion-coated systems
- 5.4.1.3 Thermal bonded structures
- 5.4.2 Coextruded barriers
- 5.4.2.1 Multi-layer coextrusion
- 5.4.2.2 Tie-layer technologies
- 5.4.2.3 Performance optimization
- 5.4.1 Laminated structures
- 5.5 Advanced barrier technologies
- 5.5.1 Nanotechnology-enhanced barriers
- 5.5.2 Plasma-deposited coatings
- 5.5.3 Atomic layer deposition systems
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 (USD Billion) (Kilo Tons)
- 6.1 Key trends
- 6.2 Solid dosage forms
- 6.2.1 Tablets and caplets
- 6.2.1.1 Immediate-release formulations
- 6.2.1.2 Extended-release systems
- 6.2.1.3 Specialty tablet applications
- 6.2.2 Capsules
- 6.2.2.1 Hard gelatin capsules
- 6.2.2.2 Soft gelatin capsules
- 6.2.2.3 Enteric-coated capsules
- 6.2.3 Powders and granules
- 6.2.3.1 Bulk powder packaging
- 6.2.3.2 Unit-dose sachets
- 6.2.3.3 Reconstitution products
- 6.2.1 Tablets and caplets
- 6.3 Liquid dosage forms
- 6.3.1 Oral liquids
- 6.3.1.1 Syrups and suspensions
- 6.3.1.2 Solutions and emulsions
- 6.3.1.3 Pediatric formulations
- 6.3.2 Injectable solutions
- 6.3.2.1 Vials and ampoules
- 6.3.2.2 Prefilled syringes
- 6.3.2.3 IV bags and containers
- 6.3.3 Topical formulations
- 6.3.3.1 Creams and ointments
- 6.3.3.2 Gels and lotions
- 6.3.3.3 Transdermal systems
- 6.3.1 Oral liquids
- 6.4 Biologics and biosimilars
- 6.4.1 Protein therapeutics
- 6.4.1.1 Monoclonal antibodies
- 6.4.1.2 Vaccines
- 6.4.1.3 Enzyme therapies
- 6.4.2 Cell and gene therapies
- 6.4.2.1 Living cell products
- 6.4.2.2 Gene therapy vectors
- 6.4.2.3 Regenerative medicine
- 6.4.1 Protein therapeutics
- 6.5 Specialty pharmaceuticals
- 6.5.1 Controlled substances
- 6.5.2 Orphan drugs
- 6.5.3 Personalized medicine
- 6.5.4 Clinical trial materials
Chapter 7 Market Estimates and Forecast, By Packaging Format, 2021 - 2034 (USD Billion) (Kilo Tons)
- 7.1 Key trends
- 7.2 Blister packaging
- 7.2.1 Thermoformed blisters
- 7.2.1.1 Pvc-based blister systems
- 7.2.1.2 Pvdc-coated blisters
- 7.2.1.3 Aluminum-aluminum blisters
- 7.2.2 Cold-formed blisters
- 7.2.2.1 Aluminum-based systems
- 7.2.2.2 High-barrier performance
- 7.2.2.3 Specialty applications
- 7.2.3 Specialty blister formats
- 7.2.3.1 Child-resistant blisters
- 7.2.3.2 Senior-friendly designs
- 7.2.3.3 Unit-dose systems
- 7.2.1 Thermoformed blisters
- 7.3 Flexible packaging
- 7.3.1 Pouches and sachets
- 7.3.1.1 Single-dose pouches
- 7.3.1.2 Multi-dose packaging
- 7.3.1.3 Stick packs
- 7.3.2 Bags and wraps
- 7.3.2.1 Bulk packaging solutions
- 7.3.2.2 Protective wrapping
- 7.3.2.3 Intermediate packaging
- 7.3.1 Pouches and sachets
- 7.4 Rigid packaging
- 7.4.1 Bottles and containers
- 7.4.1.1 Plastic bottle systems
- 7.4.1.2 Glass container applications
- 7.4.1.3 Barrier-enhanced closures
- 7.4.2 Vials and ampoules
- 7.4.2.1 Glass vial systems
- 7.4.2.2 Plastic vial applications
- 7.4.2.3 Specialized closures
- 7.4.1 Bottles and containers
- 7.5 Specialized packaging formats
- 7.5.1 Prefilled syringes
- 7.5.2 Cartridge systems
- 7.5.3 Inhalation devices
- 7.5.4 Transdermal patches
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 (USD Billion) (Kilo Tons)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Rest of Europe
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.4.6 Rest of Asia Pacific
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.5.4 Rest of Latin America
- 8.6 Middle East and Africa
- 8.6.1 Saudi Arabia
- 8.6.2 South Africa
- 8.6.3 UAE
- 8.6.4 Rest of Middle East and Africa
Chapter 9 Company Profiles
- 9.1 Amcor Plc
- 9.2 Constantia Flexibles Group GmbH
- 9.3 Klockner Pentaplast Group
- 9.4 Tekni-Plex, Inc
- 9.5 Uflex Limited
- 9.6 Mondi Group
- 9.7 Sealed Air Corporation
- 9.8 Winpak Ltd
- 9.9 Huhtamaki Oyj
- 9.10 Berry Global Inc
- 9.11 CCL Industries Inc
- 9.12 Schott AG
- 9.13 Gerresheimer AG
- 9.14 West Pharmaceutical Services, Inc
- 9.15 Sonoco Products Company











