 |
市場調查報告書
商品編碼
1773386
NUT 中線癌治療市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測NUT Midline Carcinoma Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球NUT中線癌治療市場規模達202億美元,預計到2034年將以10.7%的複合年成長率成長,達到552億美元。由於NMC在全球的盛行率不斷上升,以及診斷和治療技術的進步,該市場正在迅速擴張。政府扶持政策、臨床試驗資金以及旨在對抗罕見癌症的措施等關鍵因素正在推動這一成長。
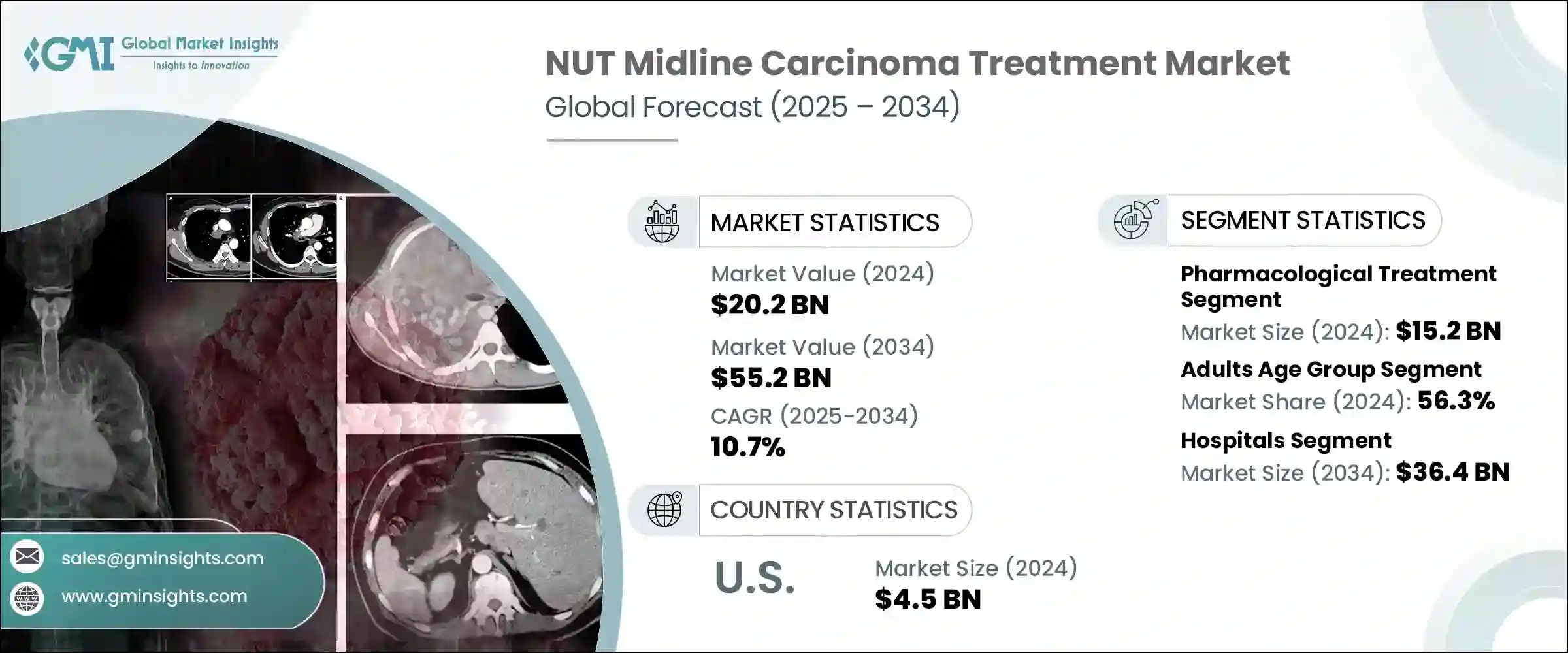
此外,先進診斷工具的普及有助於醫療專業人員更早發現NMC,從而提高治療率。此外,新興市場的城市化、醫療保健可近性的改善以及可支配收入的提高也提高了診斷率,進一步推動了市場擴張。政府和非政府來源的研究經費增加,以及對標靶療法等新型療法開發的持續關注,也對市場成長發揮重要作用。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 202億美元 |
| 預測值 | 552億美元 |
| 複合年成長率 | 10.7% |
專科腫瘤醫院和先進診斷中心數量的不斷成長,顯著促進了NUT中線癌的早期發現和改善管理,增強了市場的整體發展勢頭。這些機構擴大配備了分子影像、精準活體組織切片系統和基因定序工具等先進技術,使臨床醫生能夠更準確地識別複雜的基因異常。這種診斷精準度不僅加速了標靶治療的啟動,還改善了預後,這對於像NMC這樣的罕見且侵襲性癌症來說是一個關鍵因素。
2024年,藥物治療領域產值達152億美元。分子診斷技術的進步,例如新一代定序 (NGS) 和螢光原位雜合技術 (FISH),使得能夠精確識別NUTM1基因重排,從而實現更有針對性的藥物治療。此外,NMC的日益普及、診斷能力的提升以及對改進治療方案的需求不斷成長,正在推動市場成長。該領域也受益於新型療法的開發,例如BET抑制劑和靶向BRD-NUT融合蛋白的藥物,這些藥物在早期試驗中顯示出良好的療效。
2024年,成人市場佔最大佔有率,達56.3%。雖然NMC最初被認為主要影響兒童和青少年,但最近的研究表明,成年人,尤其是20至50歲人口的發病率更高。這種人口結構的變化,加上醫療保健和分子分析工具的普及,使得成人診斷更加便利、準確。這反過來又提高了治療的接受度,尤其是BET抑制劑和免疫療法等先進療法。
2024年,美國NUT中線癌症治療市場規模達45億美元。美國進行了許多針對罕見癌症(包括NMC)的臨床試驗。領先的機構正引領標靶療法的開發,例如BET抑制劑和免疫療法。此外,美國食品藥物管理局(FDA)已授予多種NMC療法孤兒藥資格,並提供稅收抵免、市場獨佔權和加速核准途徑等激勵措施。這些激勵措施鼓勵製藥公司加大對NMC研發的投資,進一步推動市場成長。
NUT 中線癌治療市場的主要參與者包括默克公司、C4 Therapeutics、Constellation Pharmaceuticals、輝瑞公司、Syndax Pharmaceuticals、百時美施貴寶公司、葛蘭素史克公司、羅氏公司、益普生生物製藥公司和 OncoFusion Therapeutics。 NMC 治療市場的公司致力於透過大力投資研發來鞏固其市場地位,以開發能夠解決 NMC 分子複雜性的標靶療法。
與學術和研究機構的合作使這些公司能夠加快臨床試驗,而採用先進的診斷技術則有助於他們更深入地了解疾病。此外,各公司正專注於孤兒藥地位和快速核准等監管途徑,以便更快地將新療法推向市場。此外,與擁有創新治療方案的小型生物技術公司建立合作夥伴關係並進行策略性收購,正在幫助大型企業擴大其產品組合,並為NMC(神經膠質母細胞瘤)的治療方案提供多樣化選擇。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 供應商格局
- 每個階段的增值
- 影響價值鏈的因素
- 產業衝擊力
- 成長動力
- 偵測 NMC 的先進診斷技術
- 醫院和專科腫瘤中心不斷擴張
- 治療方式不斷進步
- 產業陷阱與挑戰
- 治療費用高昂
- 治療選擇有限
- 市場機會
- 越來越關注標靶治療
- 增加臨床試驗的投資
- 成長動力
- 成長潛力分析
- 監管格局
- 北美洲
- 歐洲
- 亞太地區
- 技術格局
- 未來市場趨勢
- 管道分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 公司市佔率分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係與協作
- 新產品發布
第5章:市場估計與預測:按治療類型,2021 - 2034 年
- 主要趨勢
- 藥物治療
- 透過治療
- 化療
- 免疫療法
- 其他類型
- 依給藥途徑
- 口服
- 腸外
- 透過治療
- 非藥物治療
第6章:市場估計與預測:按年齡層,2021 - 2034 年
- 主要趨勢
- 兒科
- 成年人
第7章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 醫院
- 專科腫瘤診所
- 居家照護環境
- 其他最終用途
第8章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- Bristol-Myers Squibb Company
- C4 Therapeutics
- Constellation Pharmaceuticals
- F. Hoffmann-La Roche
- GlaxoSmithKline
- Ipsen Biopharmaceuticals
- Merck & Co
- OncoFusion Therapeutics
- Pfizer
- Syndax Pharmaceuticals
The Global NUT Midline Carcinoma Treatment Market was valued at USD 20.2 billion in 2024 and is estimated to grow at a CAGR of 10.7% to reach USD 55.2 billion by 2034. The market is expanding rapidly due to the rising global prevalence of NMC, coupled with advancements in diagnostic and therapeutic technologies. Key factors such as supportive government policies, funding for clinical trials, and initiatives aimed at combating rare cancers are driving this growth.

Moreover, the increased availability of advanced diagnostic tools is helping healthcare professionals detect NMC earlier, contributing to higher treatment uptake. Additionally, urbanization, improved healthcare access, and higher disposable incomes in emerging markets are enhancing diagnostic rates, further fueling market expansion. Increased research funding from both governmental and non-governmental sources, along with the continuous focus on developing novel treatments like targeted therapies, are also playing significant roles in market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $20.2 Billion |
| Forecast Value | $55.2 Billion |
| CAGR | 10.7% |
The growing number of specialized oncology hospitals and advanced diagnostic centers is significantly contributing to earlier detection and improved management of NUT midline carcinoma, reinforcing the market's overall momentum. These facilities are increasingly equipped with state-of-the-art technologies like molecular imaging, precision biopsy systems, and genetic sequencing tools that allow clinicians to pinpoint complex genetic abnormalities with higher accuracy. This diagnostic precision not only accelerates the initiation of targeted treatments but also enhances prognosis, which is a critical factor in rare and aggressive cancers like NMC.
The pharmacological treatment segment generated USD 15.2 billion in 2024. Technological advancements in molecular diagnostics, such as next-generation sequencing (NGS) and fluorescence in situ hybridization (FISH), have enabled precise identification of NUTM1 gene rearrangements, allowing for more targeted pharmacological treatments. Furthermore, the growing prevalence of NMC, increased diagnostic capabilities, and the rising demand for improved therapeutics options are boosting market growth. The segment is also benefitting from the development of novel therapies such as BET inhibitors and agents targeting BRD-NUT fusion proteins, which are showing promising results in early-stage trials.
In 2024, the adult segment accounted for the largest market share, with 56.3%. While NMC was initially thought to affect primarily children and adolescents, more recent studies have shown a higher incidence among adults, particularly those aged 20 to 50. This demographic shift, combined with better access to healthcare and molecular profiling tools, has led to earlier and more accurate diagnoses in adults. This, in turn, has resulted in higher treatment uptake, particularly for advanced therapies like BET inhibitors and immunotherapies.
U.S. NUT Midline Carcinoma Treatment Market was valued at USD 4.5 billion in 2024. The country is home to numerous clinical trials focused on rare cancers, including NMC. Leading institutions are spearheading the development of targeted therapies like BET inhibitors and immunotherapies. Furthermore, the U.S. FDA has granted orphan drug designations to several NMC treatments, offering incentives like tax credits, market exclusivity, and accelerated approval pathways. These incentives are encouraging pharmaceutical companies to increase their investment in NMC research and development, further propelling market growth.
Key players in the NUT Midline Carcinoma Treatment Market include Merck & Co, C4 Therapeutics, Constellation Pharmaceuticals, Pfizer, Syndax Pharmaceuticals, Bristol-Myers Squibb Company, GlaxoSmithKline, F. Hoffmann-La Roche, Ipsen Biopharmaceuticals, OncoFusion Therapeutics. Companies in the NMC treatment market focus on strengthening their position by investing heavily in research and development to create targeted therapies that address the molecular intricacies of NMC.
Collaborations with academic and research institutions allow these companies to accelerate clinical trials, while the adoption of advanced diagnostic technologies helps them gain a deeper understanding of the disease. Furthermore, companies are focusing on regulatory pathways like orphan drug status and fast-track approval to bring new treatments to market faster. Additionally, partnerships and strategic acquisitions of smaller biotech firms with innovative therapeutic solutions are helping larger players expand their product portfolios and diversify treatment options for NMC.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Disease type
- 2.2.3 Treatment type
- 2.2.4 Age group
- 2.2.5 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Availability of advanced diagnostic techniques to detect NMC
- 3.2.1.2 Increasing expansion of hospitals and specialty oncology centers
- 3.2.1.3 Growing advancement in treatment modalities
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of treatment
- 3.2.2.2 Limited treatment options
- 3.2.3 Market opportunities
- 3.2.3.1 Growing focus on targeted therapies
- 3.2.3.2 Increasing investment in clinical trials
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Pipeline analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
Chapter 5 Market Estimates and Forecast, By Treatment Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Pharmacological treatment
- 5.2.1 By therapy
- 5.2.1.1 Chemotherapy
- 5.2.1.2 Immunotherapy
- 5.2.1.3 Other types
- 5.2.2 By route of administration
- 5.2.2.1 Oral
- 5.2.2.2 Parenteral
- 5.2.1 By therapy
- 5.3 Non-pharmacological treatment
Chapter 6 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Pediatric
- 6.3 Adults
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Specialty oncology clinics
- 7.4 Homecare settings
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Bristol-Myers Squibb Company
- 9.2 C4 Therapeutics
- 9.3 Constellation Pharmaceuticals
- 9.4 F. Hoffmann-La Roche
- 9.5 GlaxoSmithKline
- 9.6 Ipsen Biopharmaceuticals
- 9.7 Merck & Co
- 9.8 OncoFusion Therapeutics
- 9.9 Pfizer
- 9.10 Syndax Pharmaceuticals






