 |
市場調查報告書
商品編碼
1773349
一次性注射器市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Disposable Syringe Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球一次性注射器市場規模達163億美元,預估年複合成長率為6.4%,2034年將達300億美元。這一成長主要源於糖尿病、心血管疾病和自體免疫疾病等慢性疾病日益加重的負擔,這些疾病都需要頻繁注射治療。此外,現代生活方式導致的健康併發症(例如肥胖和高血壓)增多,也刺激了對安全、衛生和精準給藥工具的需求。一次性注射器在降低交叉污染風險和確保精準給藥方面發揮著至關重要的作用,尤其對於需要長期治療的患者而言。
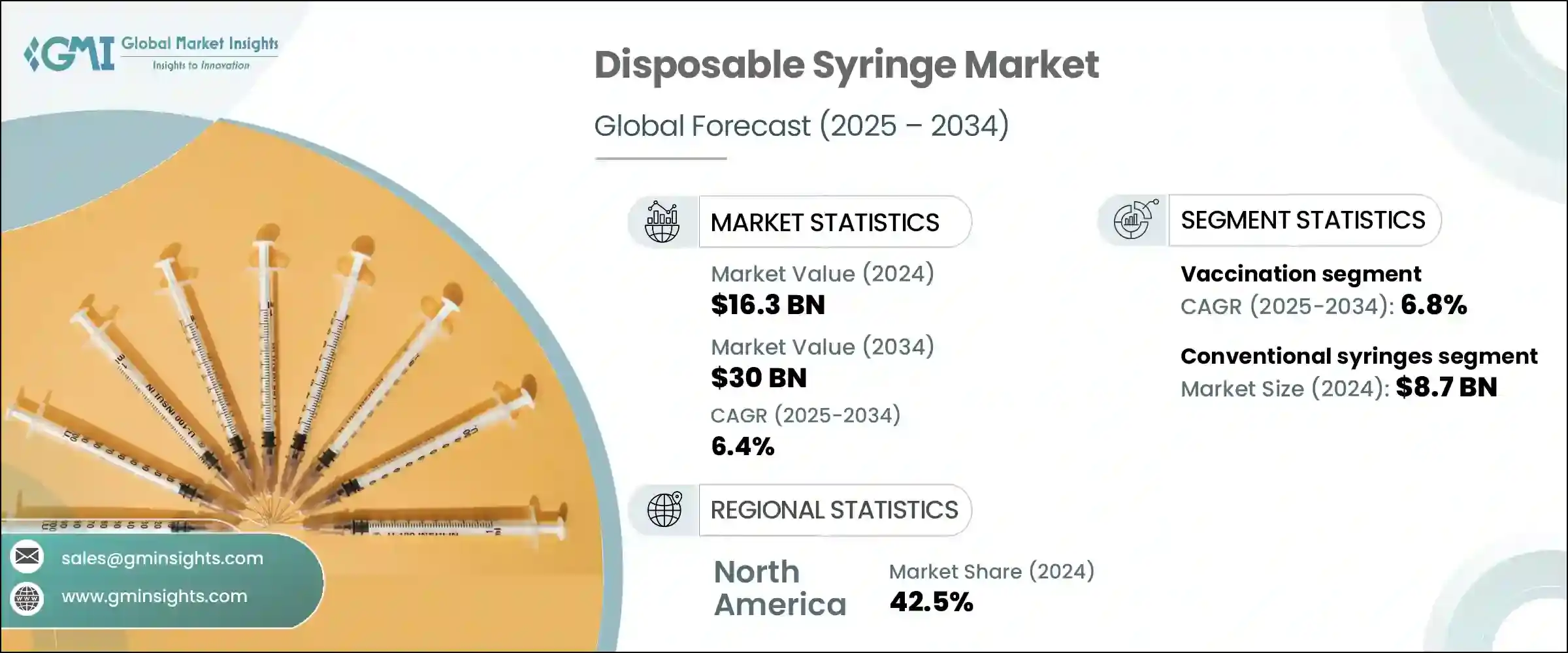
全球擴大免疫覆蓋率的推動,以及傳染病的頻傳,進一步加劇了一次性注射器在醫療基礎設施中的使用。可伸縮針頭和防針刺設計等增強安全性功能的出現,正在持續重塑診所、醫院和急救服務的採購模式。材料和注射器機制的快速改善與嚴格的醫療法規緊密結合,影響採購決策。這些因素共同為已開發經濟體和發展中經濟體一次性注射器的普及創造了良好的環境。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 163億美元 |
| 預測值 | 300億美元 |
| 複合年成長率 | 6.4% |
2024年,傳統注射器市場規模達87億美元。其結構簡單、普及性強且經濟高效,使其成為醫療和家庭護理領域中應用最廣泛的產品。這些一次性注射器常用於接種疫苗、抽血和靜脈注射藥物。由於價格實惠且易於批量採購,不同地區的政府和私人醫療機構更青睞傳統注射器。它們是持續進行的醫療保健活動、大規模疫苗接種計劃和公共衛生推廣計畫的重要組成部分。其持續的相關性可以歸因於其通用相容性、使用簡單性以及在緊急護理和常規護理中的可靠性。
預計到2034年,疫苗接種應用領域的複合年成長率將達到6.8%。全球免疫接種工作的不斷擴大推動了一次性注射器的需求成長,因為每劑疫苗通常都需要一支無菌的獨立注射器。新型疫苗的推出,包括升級的加強劑和兒科聯合疫苗,提高了疫苗接種的頻率。預防性醫療保健的這種成長趨勢極大地促進了一次性注射器使用量的增加。已開發經濟體和新興經濟體的公共衛生計畫正在透過擴大覆蓋範圍來提高注射器的使用率,從而推動供應鏈的持續活躍和製造業產量的提升。
2024年,美國一次性注射器市場規模達65億美元。美國先進的醫療體系,加上人們對醫療相關損傷日益成長的認知,使得一次性注射器成為臨床實踐的首選。需求成長的主要動力源自於人們對醫護人員安全的日益重視,尤其是在從緊急醫療服務到美容手術等各種環境中,避免意外針頭相關的損傷。此類事件的高發性凸顯了一次性注射器(尤其是配備安全機制的注射器)作為最大程度降低職業危害和遵守法規規範的重要手段。
一次性注射器市場的領導者包括 Braun Medical、Becton、Dickinson and Company、Baxter International、Terumo Corporation、Medtronic、Nipro Corporation、Agilent Technologies、Chris Merchant、Henke-Sass、Wolf、Ultimed、Vita Needle Company、Hindustan Syringes & Medical Devi、Kohfsun、Kohn 和benius Ksunnect。為了鞏固其在競爭激烈的一次性注射器領域中的地位,主要製造商正專注於產品多樣化和產品組合擴展,以滿足日益成長的全球醫療保健需求。該公司正在透過採用可伸縮針頭和自動禁用機制等技術來提高注射器的安全性,以遵守嚴格的醫療法規並降低針刺風險。與衛生部、國際衛生組織和採購機構的策略合作正在增強全球分銷能力。許多公司正在增加對自動化生產線的投資,以提高效率、保持無菌並滿足公共衛生緊急情況下的數量激增。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 供應商格局
- 每個階段的增值
- 影響價值鏈的因素
- 產業衝擊力
- 成長動力
- 慢性病盛行率不斷上升
- 傳染病疫情日益增多
- 注射器設計和材料的技術進步
- 外科手術和門診量不斷增加
- 產業陷阱與挑戰
- 安全注射器成本高
- 塑膠醫療廢棄物引發的環境問題
- 市場機會
- 轉向可生物分解和環保的注射器材料
- 預充式注射器需求不斷成長
- 成長動力
- 成長潛力分析
- 監管格局
- 波特的分析
- PESTLE 分析
- 技術和創新格局
- 當前的技術趨勢
- 新興技術
- 未來市場趨勢
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 按地區
- 北美洲
- 歐洲
- 亞太地區
- 按地區
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
- 關鍵進展
- 併購
- 夥伴關係和合作
- 新產品發布
- 擴張計劃
第5章:市場估計與預測:按產品,2021 - 2034 年
- 主要趨勢
- 傳統注射器
- 安全注射器
- 可伸縮安全注射器
- 不可伸縮安全注射器
第6章:市場估計與預測:按應用,2021 - 2034 年
- 主要趨勢
- 疫苗接種
- 血液收集
- 藥物輸送
- 其他應用
第7章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 醫院和診所
- 診斷實驗室
- 門診手術中心(ASC)
- 其他最終用途
第8章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- Agilent Technologies
- B Braun Melsungen
- Baxter International
- Becton, Dickinson and Company
- Braun Medical
- Chris Merchant
- Fresenius Kabi
- Henke-Sass, Wolf
- Hindustan Syringes & Medical Devices
- Kohope Medical
- Medtronic
- Nipro Corporation
- Terumo Corporation
- Ultimed
- Vita Needle Company
The Global Disposable Syringe Market was valued at USD 16.3 billion in 2024 and is estimated to grow at a CAGR of 6.4% to reach USD 30 billion by 2034. This growth is largely driven by the increasing burden of chronic illnesses, including diabetes, cardiovascular conditions, and autoimmune diseases, all of which require frequent injectable therapies. Additionally, a rise in health complications associated with modern lifestyles-such as obesity and hypertension-is fueling demand for safe, hygienic, and precise drug delivery tools. Disposable syringes play a vital role in minimizing cross-contamination risks and ensuring accurate dosing, especially in patients requiring long-term treatments.

The global push for expanded immunization coverage, along with frequent outbreaks of infectious diseases, has further intensified the use of disposable syringes across healthcare infrastructures. The emergence of safety-enhanced features like retractable needles and anti-needlestick designs continues to reshape the procurement patterns in clinics, hospitals, and emergency services. Rapid improvements in materials and syringe mechanisms are aligning closely with stringent healthcare regulations, influencing purchasing decisions. These collective factors are creating a robust landscape for single-use syringe adoption across both developed and developing economies.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $16.3 Billion |
| Forecast Value | $30 Billion |
| CAGR | 6.4% |
In 2024, the conventional syringes segment generated USD 8.7 billion. Their straightforward construction, widespread availability, and cost-efficiency make them the most widely used across medical and home care settings. These single-use syringes are favored for administering vaccines, drawing blood, and delivering intravenous medications. Governments and private medical facilities across various regions prefer conventional syringes due to their affordability and ease of bulk procurement. They are a critical component in ongoing healthcare campaigns, large-scale vaccination initiatives, and public health outreach programs. Their continued relevance can be attributed to their universal compatibility, simplicity in use, and reliability in both emergency and routine care.
The vaccination application segment is expected to grow at a CAGR of 6.8% through 2034. Expanding global immunization efforts are driving increased demand for single-use syringes, as each administered vaccine dose typically requires a sterile, individual syringe. The rollout of newer vaccines, including updated booster shots and pediatric combination formulas, has led to a higher frequency of vaccination drives. This growing trend in preventive healthcare significantly contributes to the rising use of disposable syringes. Public health initiatives across both developed and emerging economies are amplifying syringe utilization through expanded outreach, thereby pushing continuous supply chain activity and heightened manufacturing output.
United States Disposable Syringe Market was valued at USD 6.5 billion in 2024. The country's advanced healthcare system, combined with rising awareness around healthcare-associated injuries, has positioned disposable syringes as a preferred option in clinical practice. A major driver of demand stems from the increasing focus on healthcare worker safety, especially to avoid accidental needle-related injuries in environments ranging from emergency medical services to aesthetic procedures. The high incidence of these events underlines the importance of single-use syringes, especially those designed with safety mechanisms, as a means of minimizing occupational hazards and adhering to regulatory protocols.
Leading players in the Disposable Syringe Market include Braun Medical, Becton, Dickinson and Company, Baxter International, Terumo Corporation, Medtronic, Nipro Corporation, Agilent Technologies, Chris Merchant, Henke-Sass, Wolf, Ultimed, Vita Needle Company, Hindustan Syringes & Medical Devices, Kohope Medical, Fresenius Kabi, and B Braun Melsungen. To reinforce their presence in the competitive disposable syringe landscape, key manufacturers are focusing on product diversification and portfolio expansion that addresses growing global healthcare demands. Companies are advancing syringe safety by incorporating technologies such as retractable needles and auto-disable mechanisms to comply with strict medical regulations and reduce needlestick risks. Strategic collaborations with health ministries, international health organizations, and procurement agencies are enhancing global distribution capabilities. Many firms are increasing investments in automated production lines to boost efficiency, maintain sterility, and meet volume surges during public health emergencies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360º synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Product type
- 2.2.3 Filament
- 2.2.4 Application
- 2.2.5 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of chronic diseases
- 3.2.1.2 Growing number of infectious disease outbreak
- 3.2.1.3 Technological advancements in syringe design and material
- 3.2.1.4 Rising surgical procedures and outpatient care volume
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of safety syringes
- 3.2.2.2 Environmental concerns due to plastic medical waste
- 3.2.3 Market opportunities
- 3.2.3.1 Shift towards biodegradable and eco-friendly syringe materials
- 3.2.3.2 Rising demand for prefilled syringes
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.5 Porter's analysis
- 3.6 PESTLE analysis
- 3.7 Technology and innovation landscape
- 3.7.1 Current technological trends
- 3.7.2 Emerging technologies
- 3.8 Future market trends
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 By region
- 4.2.1.1 North America
- 4.2.1.2 Europe
- 4.2.1.3 Asia Pacific
- 4.2.1 By region
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
- 4.7 Key developments
- 4.7.1 Mergers and acquisitions
- 4.7.2 Partnerships and collaborations
- 4.7.3 New product launches
- 4.7.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Conventional syringes
- 5.3 Safety syringes
- 5.3.1 Retractable safety syringes
- 5.3.2 Non-retractable safety syringes
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Vaccination
- 6.3 Blood collection
- 6.4 Drug delivery
- 6.5 Other applications
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals and clinics
- 7.3 Diagnostic laboratories
- 7.4 Ambulatory surgical centers (ASCs)
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profile
- 9.1 Agilent Technologies
- 9.2 B Braun Melsungen
- 9.3 Baxter International
- 9.4 Becton, Dickinson and Company
- 9.5 Braun Medical
- 9.6 Chris Merchant
- 9.7 Fresenius Kabi
- 9.8 Henke-Sass, Wolf
- 9.9 Hindustan Syringes & Medical Devices
- 9.10 Kohope Medical
- 9.11 Medtronic
- 9.12 Nipro Corporation
- 9.13 Terumo Corporation
- 9.14 Ultimed
- 9.15 Vita Needle Company










