 |
市場調查報告書
商品編碼
1773272
真皮再生市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Dermal Regeneration Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球真皮再生市場規模達10.1億美元,預計到2034年將以7.7%的複合年成長率成長,達到21億美元。這一成長主要源於對非侵入性美容治療需求的不斷成長、燒傷病例的不斷增加以及糖尿病足潰瘍、下肢靜脈潰瘍和壓瘡等慢性傷口的高發生率。由於全球糖尿病患者人數的不斷成長和人口老化,這些疾病正變得越來越普遍,從而推動了對先進傷口護理療法的需求。這些複雜傷口發生率的不斷上升,迫切需要先進的真皮再生解決方案。
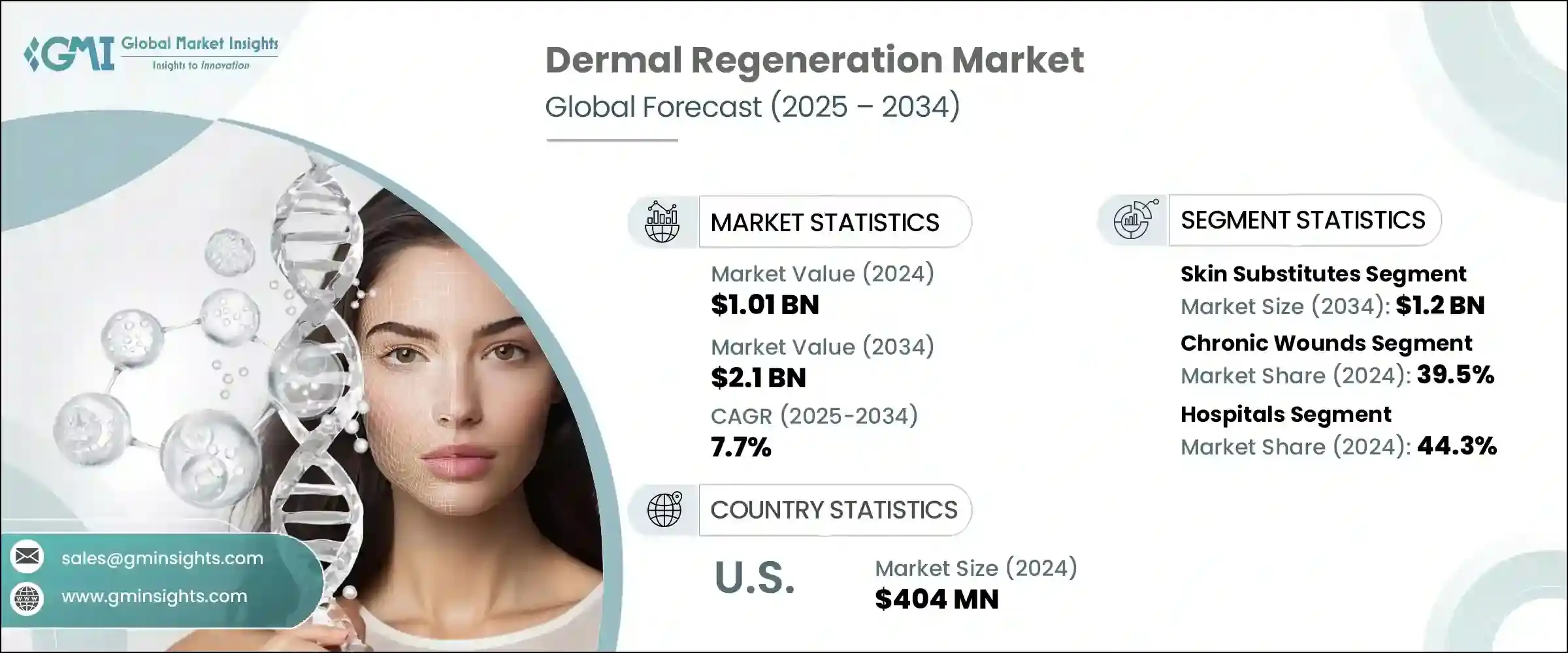
此外,包括生物工程皮膚替代品、支架和基於生長因子的療法在內的創新治療方案的進步,也支持了市場的進一步成長。醫療保健資金的增加、人們對先進治療益處的認知不斷提高以及支持性報銷政策的訂定,都有助於維持並加速全球對真皮再生產品的需求。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 10.1億美元 |
| 預測值 | 21億美元 |
| 複合年成長率 | 7.7% |
真皮再生是指利用各種方法(例如真皮替代品、支架以及細胞或非細胞基質)來恢復皮膚完整性的重建過程。這些產品經過專門設計,可透過促進組織整合、血管重建和有效傷口閉合,幫助癒合慢性傷口、燒傷、創傷和重建手術。
2024年,皮膚替代品領域以5.973億美元的估值領先市場,預計到2034年將達到12億美元,複合年成長率為7.5%。該領域包括合成和生物皮膚替代品。與傳統移植相比,它們廣泛應用於慢性傷口、燒傷和手術傷口的治療,透過降低感染風險和疤痕形成來改善癒合效果。臨床醫生青睞這些替代品,因為它們在治療糖尿病足潰瘍和大面積燒傷等複雜疾病方面效果顯著。 FDA批准數量的增加、強力的臨床證據以及完善的報銷框架(尤其是在已開發地區)進一步支持了該領域的成長。
慢性傷口領域在2024年佔據了最大的市場佔有率,達到39.5%,預計在整個預測期內將保持強勁成長。此領域包括糖尿病足潰瘍、腿部靜脈潰瘍和壓瘡。傳統的傷口癒合方法往往面臨挑戰,尤其是在糖尿病患者中,這增加了對創新皮膚替代品和再生療法的需求。慢性傷口的治療需要長期全面的護理,這會顯著影響醫療成本和患者的生活品質。因此,先進的皮膚再生產品能夠提供更好的治療效果,減少住院時間和併發症。
美國真皮再生市場在2024年達到4.04億美元,預計2025年至2034年的複合年成長率將達到7.2%。美國憑藉其先進的醫療基礎設施、較高的慢性傷口發生率以及在再生醫學研究方面的大量投入,保持了其領先地位。美國廣泛採用先進的生物合成基質、細胞療法和支架,使其成為真皮再生領域的全球領導者。獲得FDA批准的專業皮膚替代品等創新產品正在推動市場發展。
真皮再生產業的領導公司包括 MiMedx、Integra LifeSciences、Avita Medical、Gunze、Smith & Nephew、Organogenic、PolyNovo、RTI Surgical、BioTissue、Extremity Care、Kerecis (Coloplast)、MedSkin Solutions Dr. Suwelack、Stryker、Zimmera 和 Biojects。為了鞏固市場地位,真皮再生領域的公司正大力投入研發,以創新更有效、生物相容性更強、更容易使用的產品,以滿足複雜傷口類型的需求。許多公司正在投資開發具有更高整合度和癒合性能的下一代皮膚替代品和支架技術。與醫療保健提供者和研究機構的策略合作與夥伴關係,可以加快臨床應用並擴大地域覆蓋範圍。公司還優先考慮監管部門的批准和報銷談判,以確保市場准入。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 慢性傷口及燒傷發生率上升
- 生物材料和組織工程的進展
- 美容和整形手術的需求日益成長
- 政府支持和報銷政策
- 產業陷阱與挑戰
- 先進產品成本高
- 監管和核准挑戰
- 市場機會
- 智慧生物材料與3D生物列印的融合
- 擴大燒傷美容手術的醫療旅遊
- 成長動力
- 成長潛力分析
- 技術格局
- 監管格局
- 管道分析
- 未來市場趨勢
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 關鍵進展
- 併購
- 夥伴關係和合作
- 擴張計劃
第5章:市場估計與預測:依產品類型,2021 - 2034 年
- 主要趨勢
- 皮膚替代品
- 合成的
- 生物
- 無細胞
- 蜂巢
- 支架
- 其他產品類型
第6章:市場估計與預測:按應用,2021 - 2034 年
- 主要趨勢
- 慢性傷口
- 糖尿病足潰瘍
- 靜脈性腿部潰瘍
- 壓瘡
- 燒傷
- 急性傷口
- 整形外科
- 其他應用
第7章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 醫院
- 皮膚科和美容診所
- 專科診所
- 門診手術中心(ASC)
- 其他最終用途
第8章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- Anika Therapeutics
- Avita Medical
- BioTissue
- Extrimity Care
- Gunze
- Integra Lifesciences
- Kerecis (Coloplast)
- MedSkin Solutions Dr. Suwelack
- MiMedx
- Organogenesis
- PolyNovo
- RTI Surgical
- Smith & Nephew
- Stryker
- Tissue Regenix
- Zimmer Biomet
The Global Dermal Regeneration Market was valued at USD 1.01 billion in 2024 and is estimated to grow at a CAGR of 7.7% to reach USD 2.1 billion by 2034. This expansion is mainly fueled by the increasing demand for non-invasive cosmetic treatments, a rising number of burn injuries, and the high prevalence of chronic wounds such as diabetic foot ulcers, venous leg ulcers, and pressure ulcers. These conditions are becoming more common due to the growing diabetic population and the aging demographic worldwide, driving the need for advanced wound care therapies. The rising incidence of these complicated wounds creates a pressing demand for sophisticated dermal regeneration solutions.

Moreover, the advancement of innovative treatment options, including bioengineered skin substitutes, scaffolds, and growth factor-based therapies, supports further market growth. Increased healthcare funding, growing awareness of advanced treatment benefits, and supportive reimbursement policies all contribute to sustaining and accelerating demand for dermal regeneration products globally.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.01 Billion |
| Forecast Value | $2.1 Billion |
| CAGR | 7.7% |
Dermal regeneration involves reconstructive processes designed to restore skin integrity using various approaches such as dermal substitutes, scaffolds, and cellular, or acellular matrices. These products are specifically engineered to aid in healing chronic wounds, burns, traumatic injuries, and reconstructive surgeries by promoting tissue integration, revascularization, and effective wound closure.
In 2024, the skin substitutes segment led the market with a valuation of USD 597.3 million and is expected to reach USD 1.2 billion by 2034, growing at a CAGR of 7.5%. This segment comprises both synthetic and biological skin substitutes. Their broad application in managing chronic wounds, burns, and surgical wounds enhances healing outcomes by reducing infection risk and scarring compared to conventional grafts. Clinicians favor these substitutes for their effectiveness in treating complex conditions like diabetic foot ulcers and extensive burn injuries. The segment's growth is further supported by increasing FDA approvals, strong clinical evidence, and well-established reimbursement frameworks, especially in developed regions.
The chronic wounds segment captured the largest market share of 39.5% in 2024 and is forecasted to maintain strong growth throughout the forecast period. This segment includes diabetic foot ulcers, venous leg ulcers, and pressure ulcers. Traditional wound healing methods often face challenges, particularly in diabetic patients, which escalates the demand for innovative skin substitutes and regenerative therapies. Managing chronic wounds requires prolonged and comprehensive care, significantly affecting healthcare costs and patient quality of life. Consequently, advanced dermal regeneration products offer better therapeutic outcomes, reducing hospitalization time and complications.
U.S. Dermal Regeneration Market generated USD 404 million in 2024 and is anticipated to grow at a CAGR of 7.2% from 2025 through 2034. The U.S. maintains its leadership position due to its advanced healthcare infrastructure, high rates of chronic wound incidence, and substantial investments in regenerative medicine research. The country widely adopts sophisticated biosynthetic matrices, cellular therapies, and scaffolds, making it a global front-runner in dermal regeneration. Innovative products like specialized skin substitutes with FDA clearance are propelling market momentum.
Leading companies in the Dermal Regeneration Industry include MiMedx, Integra LifeSciences, Avita Medical, Gunze, Smith & Nephew, Organogenesis, PolyNovo, RTI Surgical, BioTissue, Extremity Care, Kerecis (Coloplast), MedSkin Solutions Dr. Suwelack, Stryker, Zimmer Biomet, and Tissue Regenix. To solidify their market presence, companies in the dermal regeneration sector are focusing heavily on research and development to innovate more effective, biocompatible, and easy-to-use products tailored to complex wound types. Many are investing in developing next-generation skin substitutes and scaffold technologies with improved integration and healing properties. Strategic collaborations and partnerships with healthcare providers and research institutions enable faster clinical adoption and expanded geographic reach. Firms also prioritize regulatory approvals and reimbursement negotiations to ensure market access.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Product type
- 2.2.3 Application
- 2.2.4 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of chronic wounds and burns
- 3.2.1.2 Advancements in biomaterials and tissue engineering
- 3.2.1.3 Growing demand for aesthetic and reconstructive procedures
- 3.2.1.4 Supportive government and reimbursement policies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of advanced products
- 3.2.2.2 Regulatory and approval challenges
- 3.2.3 Market opportunities
- 3.2.3.1 Integration of smart biomaterials and 3D bioprinting
- 3.2.3.2 Expanding medical tourism for burn and cosmetic procedures
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Technology landscape
- 3.5 Regulatory landscape
- 3.6 Pipeline analysis
- 3.7 Future market trends
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Key developments
- 4.5.1 Mergers and acquisitions
- 4.5.2 Partnerships and collaborations
- 4.5.3 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Skin substitutes
- 5.2.1 Synthetic
- 5.2.2 Biological
- 5.2.2.1 Acellular
- 5.2.2.2 Cellular
- 5.3 Scaffolds
- 5.4 Other product types
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Chronic wounds
- 6.2.1 Diabetic foot ulcers
- 6.2.2 Venous leg ulcers
- 6.2.3 Pressure ulcers
- 6.3 Burn injuries
- 6.4 Acute wounds
- 6.5 Plastic and reconstructive surgery
- 6.6 Other applications
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Dermatology and aesthetic clinics
- 7.4 Specialty clinics
- 7.5 Ambulatory surgical centers (ASCs)
- 7.6 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Anika Therapeutics
- 9.2 Avita Medical
- 9.3 BioTissue
- 9.4 Extrimity Care
- 9.5 Gunze
- 9.6 Integra Lifesciences
- 9.7 Kerecis (Coloplast)
- 9.8 MedSkin Solutions Dr. Suwelack
- 9.9 MiMedx
- 9.10 Organogenesis
- 9.11 PolyNovo
- 9.12 RTI Surgical
- 9.13 Smith & Nephew
- 9.14 Stryker
- 9.15 Tissue Regenix
- 9.16 Zimmer Biomet







