 |
市場調查報告書
商品編碼
1755288
VMAT2 抑制劑市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測VMAT2 Inhibitors Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
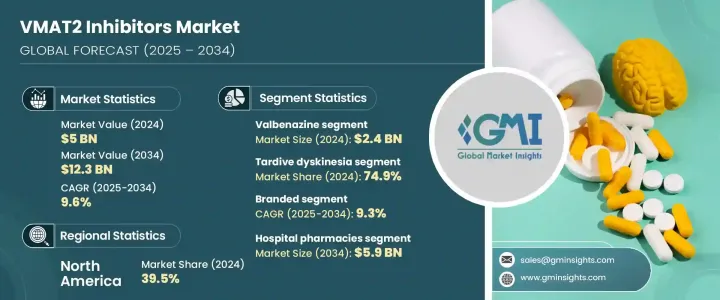
2024年,全球VMAT2抑制劑市場規模達50億美元,預計到2034年將以9.6%的複合年成長率成長,達到123億美元。這主要得益於運動相關神經系統疾病發生率的不斷上升,尤其是遲發性運動障礙(TD)和亨丁頓舞蹈症(HD)。這些疾病需要標靶治療來控制運動症狀,增加了對這類藥物的依賴。此外,耐受性更好、療效更好的VMAT2抑制劑的推出,加上更廣泛的監管批准和持續的研發投入,正在持續推動市場發展。製藥公司正在利用商業策略、政策支持和擴大患者覆蓋範圍,以提高藥物的可及性並深化市場覆蓋。
VMAT2抑制劑透過降低多巴胺、血清素和去甲腎上腺素等神經傳導物質的水平發揮作用,有助於治療過動症。這些藥物阻斷單胺類物質向突觸囊泡的運輸,有助於調節導致不自主運動問題的過度多巴胺活性。這些療法主要用於治療亨廷頓舞蹈症 (HD) 和遲發性運動障礙 (TD) 等疾病,為控制嚴重損害患者生活品質的衰弱症狀提供了一種有希望的解決方案。透過調節大腦中過量的多巴胺活性,VMAT2抑制劑有助於控制這些疾病中常見的不自主運動、肌肉痙攣和其他運動功能障礙。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 50億美元 |
| 預測值 | 123億美元 |
| 複合年成長率 | 9.6% |
Valbenazine 板塊領先市場,2024 年估值達 24 億美元,這得益於其強大的療效、良好的安全性記錄和簡便的給藥方案,這些因素促成了高處方量。作為首批獲準用於治療 TD 的 VMAT2 抑制劑之一,Valbenazine 獲得了早期市場佔有率和品牌信譽,並擴展到更多適應症。其使用者友善的治療方案進一步提高了患者的依從性,並推動了持續的需求。
遲發性運動障礙(TD)細分市場在2024年的市佔率為74.9%,這得益於長期接受抗精神病藥物治療的精神疾病患者中TD的診斷率不斷上升。專門用於治療TD的VMAT2抑制劑的核准和上市顯著改善了治療效果。認知度的提高和篩檢工作的進行進一步促進了及時干預,從而促進了這些療法的普及。
受精神科護理普及化、先進診斷技術和完善的報銷體系的推動,美國VMAT2抑制劑市場規模在2024年達到18億美元。成熟的醫療基礎設施和病人友善的保險模式持續推動著VMAT2抑制劑在各類醫療機構的應用。精神分裂症、躁鬱症和重度憂鬱症等神經精神疾病的高診斷率和治療率顯著促進了市場需求,因為這些疾病通常與藥物引起的運動障礙(如遲發性運動障礙)有關。
全球VMAT2抑制劑市場的主要參與者包括:Neurocrine Biosciences、Teva Pharmaceutical Industries、Lupin、Dr. Reddy's Laboratories、H. Lundbeck、Intas Pharmaceuticals Ltd、Bausch Health Companies、APOTEX、Mylan、Actavis Labs、BionPharma和Upsher-Smith Laboratories。 VMAT2抑制劑市場的領先公司正致力於拓展治療應用、改進產品配方並提升生產能力。許多公司正在投資臨床試驗,以獲得監管部門對TD和HD以外其他適應症的批准。與醫療保健提供者和保險公司的策略合作也有助於提高藥物的可近性和患者依從性。 Intas、Bausch Health Companies和Neurocrine Biosciences等公司正在透過下一代療法和差異化給藥策略來增強其產品組合。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 遲發性運動障礙(TD)和亨丁頓舞蹈症的盛行率不斷上升
- 新型藥物製劑的進展
- 提高對運動障礙的認知和診斷
- 產業陷阱與挑戰
- 藥品成本高
- VMAT2抑制劑相關的副作用
- 成長動力
- 成長潛力分析
- 監管格局
- 川普政府關稅
- 對貿易的影響
- 貿易量中斷
- 報復措施
- 對產業的影響
- 供應方影響(原料)
- 主要材料價格波動
- 供應鏈重組
- 生產成本影響
- 需求面影響(售價)
- 價格傳導至終端市場
- 市佔率動態
- 消費者反應模式
- 供應方影響(原料)
- 受影響的主要公司
- 策略產業反應
- 供應鏈重組
- 定價和產品策略
- 政策參與
- 展望與未來考慮
- 對貿易的影響
- 未來市場趨勢
- 管道分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按藥物,2021-2034 年
- 主要趨勢
- 伐貝那嗪
- 氘代丁苯那嗪
- 丁苯那嗪
第6章:市場估計與預測:按應用,2021-2034
- 主要趨勢
- 遲發性運動障礙(TD)
- 亨丁頓舞蹈症
第7章:市場估計與預測:按類型,2021-2034
- 主要趨勢
- 品牌
- 通用的
第 8 章:市場估計與預測:按配銷通路,2021-2034 年
- 主要趨勢
- 醫院藥房
- 零售藥局
- 網路藥局
第9章:市場估計與預測:按地區,2021-2034
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- Actavis Labs
- APOTEX
- Bausch Health Companies
- BionPharma
- Dr. Reddy's Laboratories
- H. Lundbeck
- Intas Pharmaceuticals Ltd
- Lupin
- Mylan
- Neurocrine Biosciences
- Teva Pharmaceutical Industries
- Upsher-Smith Laboratories
The Global VMAT2 Inhibitors Market was valued at USD 5 billion in 2024 and is estimated to grow at a CAGR of 9.6% to reach USD 12.3 billion by 2034, driven by the growing incidence of movement-related neurological disorders, particularly Tardive Dyskinesia (TD) and Huntington's Disease (HD). These conditions require targeted therapies to manage motor symptoms, increasing reliance on this drug class. Additionally, introducing better-tolerated and more effective VMAT2 inhibitors, combined with broader regulatory approvals and ongoing R&D, continues to push the market forward. Pharma companies are leveraging commercial strategies, policy support, and expanding patient outreach to improve drug accessibility and deepen market reach.

VMAT2 inhibitors work by decreasing the levels of neurotransmitters like dopamine, serotonin, and norepinephrine, helping to manage hyperkinetic movement disorders. These drugs block the transport of monoamines into synaptic vesicles, helping to regulate excess dopamine activity that contributes to involuntary motor issues. Used primarily in conditions such as Huntington's Disease (HD) and Tardive Dyskinesia (TD), these therapies offer a promising solution for managing debilitating symptoms that significantly impair patients' quality of life. By regulating excessive dopamine activity in the brain, VMAT2 inhibitors help control involuntary movements, muscle spasms, and other motor dysfunctions commonly seen in these disorders.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $5 Billion |
| Forecast Value | $12.3 Billion |
| CAGR | 9.6% |
Valbenazine segment led the market with a valuation of USD 2.4 billion in 2024 supported by its strong efficacy profile, favorable safety record, and simple dosing regimen, all of which have contributed to high prescription volumes. As one of the first VMAT2 inhibitors approved for TD, it secured early market presence, brand credibility, and has since expanded its use across additional indications. Its user-friendly treatment schedule further enhances patient compliance and drives sustained demand.
The Tardive Dyskinesia segment accounted for 74.9% share in 2024 fueled by the increasing diagnosis of TD in patients treated long-term with antipsychotics for psychiatric conditions. The approval and marketing of VMAT2 inhibitors specifically designed to address TD has significantly improved treatment outcomes. Rising awareness and screening efforts are further enabling timely intervention, contributing to the increased uptake of these therapies.
U.S. VMAT2 Inhibitors Market reached USD 1.8 billion in 2024 driven by widespread access to psychiatric care, advanced diagnostics, and strong reimbursement structures. A mature healthcare infrastructure and patient-friendly insurance models continue to facilitate the adoption of VMAT2 inhibitors across various care settings. The high rate of diagnosis and treatment for neuropsychiatric disorders such as schizophrenia, bipolar disorder, and major depressive disorder contributes significantly to market demand, as these conditions are commonly associated with drug-induced movement disorders like Tardive Dyskinesia.
Key players in the Global VMAT2 Inhibitors Market include: Neurocrine Biosciences, Teva Pharmaceutical Industries, Lupin, Dr. Reddy's Laboratories, H. Lundbeck, Intas Pharmaceuticals Ltd, Bausch Health Companies, APOTEX, Mylan, Actavis Labs, BionPharma, and Upsher-Smith Laboratories. Leading companies in the VMAT2 Inhibitors Market are focusing on expanding therapeutic applications, enhancing product formulations, and scaling up manufacturing capabilities. Many are investing in clinical trials to gain regulatory approval for additional indications beyond TD and HD. Strategic collaborations with healthcare providers and insurers are also helping to improve drug accessibility and patient adherence. Firms like Intas, Bausch Health Companies, and Neurocrine Biosciences are bolstering their portfolios with next-generation therapies and differentiated dosing strategies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of Tardive Dyskinesia (TD) and Huntington’s disease
- 3.2.1.2 Advancements in novel drug formulations
- 3.2.1.3 Increased awareness and diagnosis of movement disorders
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of drugs
- 3.2.2.2 Side effects associated with VMAT2 inhibitors
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Future market trends
- 3.7 Pipeline analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Drug, 2021-2034 ($ Mn)
- 5.1 Key trends
- 5.2 Valbenazine
- 5.3 Deutetrabenazine
- 5.4 Tetrabenazine
Chapter 6 Market Estimates and Forecast, By Application, 2021-2034 ($ Mn)
- 6.1 Key trends
- 6.2 Tardive dyskinesia (TD)
- 6.3 Huntington's disease
Chapter 7 Market Estimates and Forecast, By Type, 2021-2034 ($ Mn)
- 7.1 Key trends
- 7.2 Branded
- 7.3 Generic
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021-2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021-2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Actavis Labs
- 10.2 APOTEX
- 10.3 Bausch Health Companies
- 10.4 BionPharma
- 10.5 Dr. Reddy's Laboratories
- 10.6 H. Lundbeck
- 10.7 Intas Pharmaceuticals Ltd
- 10.8 Lupin
- 10.9 Mylan
- 10.10 Neurocrine Biosciences
- 10.11 Teva Pharmaceutical Industries
- 10.12 Upsher-Smith Laboratories



