 |
市場調查報告書
商品編碼
1750354
特發性血小板減少性紫斑症 (ITP) 治療市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
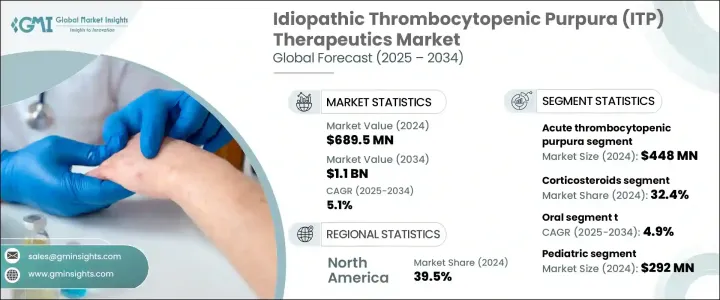
2024年,全球特發性血小板減少性紫斑症 (ITP) 治療市場規模達6.895億美元,預計到2034年將以5.1%的複合年成長率成長,達到11億美元。這主要得益於全球自體免疫疾病病例的增加,以及對ITP認知的提高和早期診斷的提升。診斷工具的技術進步使得早期檢測ITP變得更加容易。加上許多地區醫療服務可近性的提高,有助於醫療機構及時啟動治療,進而進一步刺激治療需求。
現代診斷技術,包括先進的血小板功能檢測和免疫標記分析,幫助醫護人員更好地理解和識別ITP。這些進展導致確診病例顯著增加,也增加了對可靠有效治療方法的需求。治療領域的創新,尤其是血小板生成素受體激動劑的日益普及,改善了慢性ITP患者的預後。事實證明,這些療法比傳統治療方法更有效、更容易管理。同時,活躍的藥物研發管線為治療重症和難治性ITP帶來了希望,增強了該產業的成長預期。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 6.895億美元 |
| 預測值 | 11億美元 |
| 複合年成長率 | 5.1% |
在疾病類型中,急性血小板減少性紫斑症(ITP)在2024年的市場價值為4.48億美元,這主要得益於兒童病例數量的增加,這些病例通常在病毒感染後發展為急性ITP。這些新的緊急病例需要快速的治療干預,主要依靠靜脈注射免疫球蛋白和皮質類固醇等經濟有效的治療方法。由於這些患者的出血風險較高,立即治療至關重要,因此對第一線治療藥物的需求持續穩定。
2024年,皮質類固醇市場佔有32.4%的佔有率。其在抑制免疫活性和提高血小板水平方面的有效性使其成為首選。此外,價格實惠、廣泛可用以及與成人和兒科治療方案相容也使其成為市場主導。皮質類固醇因其口服和注射劑型、給藥簡便以及被納入主要臨床指南而經常被處方。
2024年,美國特發性血小板減少性紫斑症 (ITP) 治療市場規模達2.486億美元。高發病率、早期疾病檢測以及專科護理機構的普及,共同推動了該市場的擴張。美國擁有成熟的醫療基礎設施和完善的報銷體系,確保急性和慢性ITP患者都能更廣泛地獲得先進的治療方案。正在進行的臨床試驗和學術研究也加速了下一代療法的開發,促進了生物製劑和血小板生成素受體激動劑的穩定應用。
該行業的主要參與者包括Sobi、Intas Pharmaceuticals、安進、諾華、Kedrion Biopharma、羅氏製藥、葛蘭素史克、傑富林、基立福和Octapharma AG。為了穩固立足,領先公司正在透過收購和推出新型生物製劑來擴大其產品組合。他們專注於獲得FDA和EMA批准,用於治療急性和慢性ITP的先進療法。與研究機構的策略合作推動創新,而以患者為中心的解決方案(例如自主注射劑)的投資則增強了市場競爭力。向新興醫療保健市場的全球擴張進一步支持了長期成長。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- ITP盛行率上升
- 治療方案的進展
- 提高認知和早期診斷
- 產業陷阱與挑戰
- 某些療法的不良反應
- 成長動力
- 成長潛力分析
- 監管格局
- 川普政府關稅
- 對貿易的影響
- 貿易量中斷
- 報復措施
- 對產業的影響
- 供應方影響(原料)
- 主要材料價格波動
- 供應鏈重組
- 生產成本影響
- 需求面影響(售價)
- 價格傳導至終端市場
- 市佔率動態
- 消費者反應模式
- 供應方影響(原料)
- 受影響的主要公司
- 策略產業反應
- 供應鏈重組
- 定價和產品策略
- 政策參與
- 展望與未來考慮
- 對貿易的影響
- 管道分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:依疾病類型,2021 年至 2034 年
- 主要趨勢
- 急性血小板減少性紫斑
- 慢性血小板減少性紫斑
第6章:市場估計與預測:按產品,2021 年至 2034 年
- 主要趨勢
- 皮質類固醇
- 靜脈注射免疫球蛋白(IVIG)
- 血小板生成素受體激動劑(TPO-RA)
- 其他產品
第7章:市場估計與預測:依管理路線,2021 年至 2034 年
- 主要趨勢
- 口服
- 注射劑
第8章:市場估計與預測:按年齡層,2021 年至 2034 年
- 主要趨勢
- 兒科
- 成人
- 老年
第9章:市場估計與預測:按配銷通路,2021 年至 2034 年
- 主要趨勢
- 醫院藥房
- 零售藥局
- 網路藥局
第10章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第 11 章:公司簡介
- Amgen
- Argenx
- CSL Behring
- F. Hoffmann-La Roche
- GlaxoSmithKline
- Grifols
- Intas Pharmaceuticals
- Kedrion Biopharma
- Kissei Pharmaceutical
- Novartis
- Octapharma AG
- Rigel Pharmaceuticals
- Sobi
The Global Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market was valued at USD 689.5 million in 2024 and is estimated to grow at a CAGR of 5.1% to reach USD 1.1 billion by 2034, driven by the rising cases of autoimmune disorders worldwide, along with improved awareness and early diagnosis of ITP. Technological advancements in diagnostic tools are making it easier to detect ITP in its early stages. This, combined with greater healthcare accessibility in many regions, is helping healthcare providers initiate prompt treatment, further boosting therapeutic demand.

Modern diagnostic techniques, including advanced platelet function assays and immune marker analysis, have helped healthcare professionals better understand and identify ITP. These developments lead to a noticeable rise in confirmed diagnoses, increasing the need for reliable and effective treatment methods. Innovations in therapeutics, particularly the growing use of thrombopoietin receptor agonists, improve outcomes for patients with chronic forms of the condition. These therapies are proving to be more effective and easier to manage compared to traditional treatment methods. Meanwhile, an active drug development pipeline offers hope for treating severe and refractory cases of ITP, reinforcing growth projections for the industry.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $689.5 Million |
| Forecast Value | $1.1 Billion |
| CAGR | 5.1% |
Among disease types, the acute thrombocytopenic purpura segment was valued at USD 448 million in 2024, driven by the increasing number of pediatric cases where acute ITP often develops following viral infections. These new and urgent cases require rapid therapeutic intervention, primarily relying on cost-effective treatments like intravenous immunoglobulin and corticosteroids. Immediate treatment is critical due to the heightened risk of bleeding in these patients, leading to a steady demand for first-line therapies.
The corticosteroids segment held a 32.4% share in 2024. Their effectiveness in suppressing immune activity and raising platelet levels makes them a preferred choice. Additionally, affordability, wide availability, and compatibility with adult and pediatric treatment protocols contribute to their dominance. Corticosteroids are frequently prescribed due to their oral and injectable forms, simple administration, and inclusion in major clinical guidelines.
U.S. Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market generated USD 248.6 million in 2024. The combination of a high incidence rate, early disease detection, and widespread access to specialized care facilities has supported the expansion of this market. The country benefits from a mature healthcare infrastructure and well-established reimbursement systems, which ensure broader access to advanced treatment options for both acute and chronic ITP cases. Ongoing clinical trials and academic research have also accelerated the development of next-generation therapies, contributing to the steady adoption of biologics and thrombopoietin receptor agonists.
Key players in this industry include Sobi, Intas Pharmaceuticals, Amgen, Novartis, Kedrion Biopharma, F. Hoffmann-La Roche, GlaxoSmithKline, CSL Behring, Grifols, and Octapharma AG. To secure a strong foothold, leading companies are expanding their product portfolios through acquisitions and the launch of novel biologics. They focus on securing FDA and EMA approvals for advanced therapies targeting acute and chronic ITP. Strategic collaborations with research institutions drive innovation, while investments in patient-centric solutions, such as self-administered injectables, enhance market competitiveness. Global expansion into emerging healthcare markets further supports long-term growth.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of ITP
- 3.2.1.2 Advancements in therapeutic options
- 3.2.1.3 Growing awareness and early diagnosis
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Adverse effects of certain therapies
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Pipeline analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Disease Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Acute thrombocytopenic purpura
- 5.3 Chronic thrombocytopenic purpura
Chapter 6 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Corticosteroids
- 6.3 Intravenous immunoglobulins (IVIG)
- 6.4 Thrombopoietin receptor agonists (TPO-RA)
- 6.5 Other products
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oral
- 7.3 Injectable
Chapter 8 Market Estimates and Forecast, By Age Group, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pediatric
- 8.3 Adult
- 8.4 Geriatric
Chapter 9 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospital pharmacies
- 9.3 Retail pharmacies
- 9.4 Online pharmacies
Chapter 10 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Italy
- 10.3.5 Spain
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 Japan
- 10.4.3 India
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Amgen
- 11.2 Argenx
- 11.3 CSL Behring
- 11.4 F. Hoffmann-La Roche
- 11.5 GlaxoSmithKline
- 11.6 Grifols
- 11.7 Intas Pharmaceuticals
- 11.8 Kedrion Biopharma
- 11.9 Kissei Pharmaceutical
- 11.10 Novartis
- 11.11 Octapharma AG
- 11.12 Rigel Pharmaceuticals
- 11.13 Sobi




