 |
市場調查報告書
商品編碼
1741035
C反應蛋白檢測市場機會、成長動力、產業趨勢分析及2025-2034年預測C-Reactive Protein Testing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
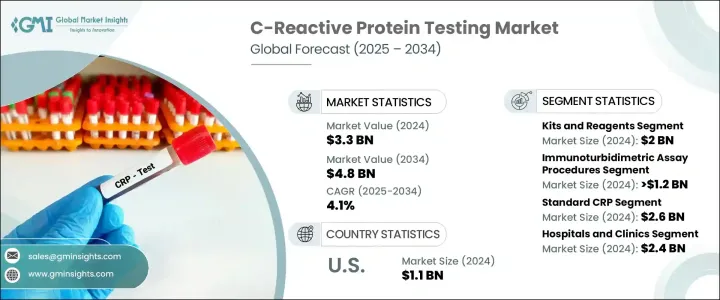
2024 年全球 C 反應蛋白檢測市場價值為 33 億美元,預計到 2034 年將以 4.1% 的複合年成長率成長至 48 億美元。慢性病盛行率的上升是推動市場成長的主要驅動力之一,因為醫療保健專業人員越來越依賴 CRP 檢測進行準確及時的診斷。常規健康篩檢和疾病管理方案中 CRP 檢測的廣泛應用也支持了這種不斷成長的需求。此測試可測量肝臟響應發炎而產生的 C 反應蛋白的濃度。其水平可能由於多種觸發因素而飆升,包括感染、發炎性疾病和心血管疾病。升高的 CRP 水平是檢測和監測體內發炎的重要標誌,可幫助醫療專業人員評估患者病情的嚴重程度並制定適當的治療策略。
隨著診斷能力的提升,CRP 檢測在全球醫療保健系統中持續受到青睞。由於其快速出結果的能力,CRP 檢測已成為早期干預不可或缺的手段,尤其是在時間緊迫的臨床環境中。 CRP 檢測在追蹤病情進展和療效方面也發揮著不可或缺的作用。 CRP 檢測的廣泛應用不僅改善了患者的預後,還提高了醫療服務的成本效益,使其成為小型診所和大型醫療機構的可靠選擇。檢測準確性、效率和易用性的提升進一步推動了市場的成長,提高了 CRP 檢測在各個醫學專科領域的實用性。因此,CRP 檢測現已被廣泛用作診斷工具,用於管理從感染、自體免疫疾病到發炎和心血管疾病等各種疾病。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 33億美元 |
| 預測值 | 48億美元 |
| 複合年成長率 | 4.1% |
就產品而言,市場細分為儀器和試劑盒及試劑。 2024年,試劑盒及試劑細分市場以20億美元的總收入領先市場。這些產品因其與多種檢測平台相容而被廣泛採用,從而能夠應用於多種檢測技術。其多功能性和快速產生結果的能力使其適用於各種臨床環境,在這些環境中,及時決策至關重要。這些試劑盒的易於整合和高效性進一步推動了它們相對於更複雜的檢測程序的青睞。
根據檢測類型,市場包括酵素連結免疫吸附試驗 (ELISA)、化學發光免疫分析 (CLIA)、免疫比濁法等。免疫比濁法在2024年佔據了最高的市場佔有率,價值超過12億美元。這類檢測因其成本效益高、週轉時間短而備受青睞,成為資源有限的小型醫療保健機構和實驗室的可行選擇。它們能夠快速提供可靠的結果,這增強了其在臨床實踐中的作用,因為快速診斷對於有效的患者管理至關重要。
根據檢測範圍,市場分為高敏感性CRP(Hs-CRP)和標準CRP。標準CRP市場在2024年佔據主導地位,預計到2034年將達到26億美元。標準CRP檢測在醫院和診斷中心廣泛用於一般發炎檢測,鞏固了其市場領先地位。其經驗證的臨床可靠性和經濟實惠性進一步推動了其持續應用。
從應用角度來看,該市場涵蓋心血管疾病、傳染病、慢性發炎性疾病及其他領域。心血管疾病領域在2024年創造了超過12億美元的收入。 CRP檢測是心血管風險評估的常見組成部分,可幫助醫療保健專業人員監測病情進展並評估治療效果。人們對心臟相關疾病的預防保健和早期干預日益重視,這支持了其在該應用領域的持續需求。
就最終用途而言,市場細分為醫院和診所、診斷實驗室和其他最終用戶。醫院和診所是2024年最大的最終用途細分市場,收入達24億美元。這些機構定期對出現發炎、感染或心血管事件症狀的患者進行CRP檢測。快速處理CRP檢測結果的能力在急診和初級保健機構中是一項關鍵優勢,因為在這些機構中,即時的臨床決策至關重要。從內科到傳染病科,CRP檢測的廣泛應用也進一步提高了其高利用率。
在美國,CRP 檢測市場在 2024 年的規模為 11 億美元,預計在預測期內將以 3.2% 的複合年成長率成長。在鼓勵早期診斷的政策支持下,預防性醫療保健的轉變正在推動 CRP 檢測在全國的普及。人們對發炎疾病的認知不斷提高以及健康教育的不斷改進也促進了醫療服務提供者和患者對檢測的使用率的提高。
約55%的市場由五家領先公司控制,其中包括持續創新並推出先進檢測解決方案的主要參與者。這些公司專注於開發經濟高效、方便用戶使用且高精度的CRP檢測產品,以滿足已開發和發展中醫療保健市場日益成長的需求。他們以技術創新和精簡診斷解決方案為策略重點,這加劇了競爭,並推動了市場成長。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 發炎性疾病等慢性疾病的盛行率不斷上升
- 用於診斷和管理慢性病的 CRP 檢測需求激增
- 技術進步
- 直接上門檢測服務的需求不斷成長
- 產業陷阱與挑戰
- 缺乏標準化
- 成長動力
- 成長潛力分析
- 監管格局
- 川普政府關稅
- 對貿易的影響
- 貿易量中斷
- 報復措施
- 對產業的影響
- 供給側影響(原料)
- 主要材料價格波動
- 供應鏈重組
- 生產成本影響
- 需求面影響(售價)
- 價格傳導至終端市場
- 市佔率動態
- 消費者反應模式
- 供給側影響(原料)
- 受影響的主要公司
- 策略產業反應
- 供應鏈重組
- 定價和產品策略
- 政策參與
- 展望與未來考慮
- 對貿易的影響
- 報銷場景
- 技術格局
- 未來市場趨勢
- 波特的分析
- 差距分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 公司市佔率分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按產品,2021 - 2034 年
- 主要趨勢
- 儀器
- 試劑盒和試劑
第6章:市場估計與預測:按檢測類型,2021 - 2034 年
- 主要趨勢
- 化學發光免疫分析(CLIA)
- 酵素連結免疫吸附試驗(ELISA)
- 免疫比濁法
- 其他檢測類型
第7章:市場估計與預測:按檢測範圍,2021 - 2034 年
- 主要趨勢
- 高敏感性CRP(hs-CRP)
- 標準CRP
第8章:市場估計與預測:按應用,2021 - 2034 年
- 主要趨勢
- 心血管疾病
- 傳染病
- 慢性發炎疾病
- 其他應用
第9章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 醫院和診所
- 診斷實驗室
- 其他最終用途
第10章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第 11 章:公司簡介
- Abbott
- Agilent
- AIDIAN
- Boditech
- Creative Diagnostics
- CTK BIOTECH
- Danaher
- DxGen Corp
- Getein Biotech
- GOLDSITE
- HORIBA
- Labcorp
- Merck
- OptiBio
- RANDOX
- Roche
- SIEMENS Healthineers
The Global C-Reactive Protein Testing Market was valued at USD 3.3 billion in 2024 and is estimated to grow at a CAGR of 4.1% to reach USD 4.8 billion by 2034. The rising prevalence of chronic conditions is one of the key drivers fueling market growth, as healthcare professionals increasingly rely on CRP tests for accurate and timely diagnosis. This growing demand is also supported by the expanding adoption of CRP testing in routine health screenings and disease management protocols. The test measures the concentration of C-reactive protein, which the liver produces in response to inflammation. Its levels can spike due to a variety of triggers, including infections, inflammatory diseases, and cardiovascular conditions. Elevated CRP levels serve as an important marker to detect and monitor inflammation in the body, assisting medical professionals in assessing the severity of a patient's condition and formulating appropriate treatment strategies.

As diagnostic capabilities evolve, CRP testing continues to gain traction in healthcare systems worldwide. With its ability to deliver rapid results, the test is becoming indispensable for early intervention, especially in clinical settings where time is critical. CRP testing has also become integral in tracking disease progression and therapeutic effectiveness. The increased use of these tests has not only improved patient outcomes but has also contributed to cost-effective care delivery, making them a reliable choice for both small clinics and large healthcare facilities. The market's growth is further supported by advancements in test accuracy, efficiency, and ease of use, which have enhanced the utility of CRP testing across various medical specialties. As a result, CRP testing is now widely used as a diagnostic tool in managing conditions ranging from infections and autoimmune diseases to inflammatory and cardiovascular disorders.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.3 Billion |
| Forecast Value | $4.8 Billion |
| CAGR | 4.1% |
In terms of products, the market is segmented into instruments and kits & reagents. In 2024, the kits and reagents segment led the market with revenue totaling USD 2 billion. These products are widely adopted due to their compatibility with diverse testing platforms, allowing their application across multiple assay techniques. Their versatility and ability to produce fast results make them suitable for various clinical settings, where timely decisions are essential. The ease of integration and efficiency of these kits further drive their preference over more complex testing procedures.
Based on assay type, the market includes enzyme-linked immunosorbent assay (ELISA), chemiluminescence immunoassay (CLIA), immunoturbidimetric assay, and others. Immunoturbidimetric assays accounted for the highest market share in 2024, reaching a value of over USD 1.2 billion. These assays are favored for their cost-effectiveness and quick turnaround times, making them an accessible choice for smaller healthcare providers and laboratories with limited resources. Their ability to deliver reliable results swiftly enhances their role in clinical practice, where fast diagnostics are essential for effective patient management.
By detection range, the market is divided into high-sensitivity CRP (Hs-CRP) and standard CRP. The standard CRP segment dominated in 2024 and is expected to reach USD 2.6 billion by 2034. The widespread use of standard CRP tests in hospitals and diagnostic centers for general inflammation detection has contributed to their market lead. Their proven clinical reliability and affordability further drive their continued use.
In terms of application, the market includes cardiovascular diseases, infectious diseases, chronic inflammatory diseases, and other uses. The cardiovascular diseases segment generated over USD 1.2 billion in revenue in 2024. CRP testing is a common component in cardiovascular risk assessment, aiding healthcare professionals in monitoring disease progression and evaluating treatment efficacy. The growing emphasis on preventive care and early intervention in heart-related conditions supports its consistent demand in this application area.
Regarding end use, the market is segmented into hospitals and clinics, diagnostic laboratories, and other end users. Hospitals and clinics were the largest end-use segment in 2024, with revenue reaching USD 2.4 billion. These facilities regularly use CRP tests for patients presenting with symptoms of inflammation, infections, or cardiovascular events. The ability to quickly process CRP test results is a key advantage in emergency and primary care settings, where immediate clinical decisions are critical. The broad scope of departments utilizing CRP testing-from internal medicine to infectious disease units-adds to its high utilization rate.
In the United States, the CRP testing market was valued at USD 1.1 billion in 2024 and is expected to grow at a CAGR of 3.2% through the forecast period. The shift toward preventive healthcare, supported by policies that encourage early diagnosis, is boosting the adoption of CRP testing across the country. Growing awareness around inflammatory conditions and better health education are also contributing to increased test usage among both healthcare providers and patients.
Approximately 55% of the market is controlled by five leading companies, including major players that continue to innovate and launch advanced testing solutions. These companies are focusing on developing cost-effective, user-friendly, and high-precision CRP testing products that meet the rising demand from both developed and developing healthcare markets. Their strategic emphasis on technological innovation and streamlined diagnostic solutions is intensifying competition and pushing market growth forward.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of chronic disorders such as inflammatory diseases
- 3.2.1.2 Surge in demand for CRP testing for diagnosis and management of chronic diseases
- 3.2.1.3 Advances in technology
- 3.2.1.4 Growing demand for direct-to-home testing services
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of standardization
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the Industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Reimbursement scenario
- 3.7 Technology landscape
- 3.8 Future market trends
- 3.9 Porter's analysis
- 3.10 GAP analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Company market share analysis
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Instruments
- 5.3 Kits and reagents
Chapter 6 Market Estimates and Forecast, By Assay Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Chemiluminescence immunoassay (CLIA)
- 6.3 Enzyme-linked immunosorbent assay (ELISA)
- 6.4 Immunoturbidimetric assay
- 6.5 Other assay types
Chapter 7 Market Estimates and Forecast, By Detection Range, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 High-sensitivity CRP (hs-CRP)
- 7.3 Standard CRP
Chapter 8 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Cardiovascular diseases
- 8.3 Infectious diseases
- 8.4 Chronic inflammatory diseases
- 8.5 Other applications
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospitals and clinics
- 9.3 Diagnostic laboratories
- 9.4 Other end uses
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 Japan
- 10.4.3 India
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Abbott
- 11.2 Agilent
- 11.3 AIDIAN
- 11.4 Boditech
- 11.5 Creative Diagnostics
- 11.6 CTK BIOTECH
- 11.7 Danaher
- 11.8 DxGen Corp
- 11.9 Getein Biotech
- 11.10 GOLDSITE
- 11.11 HORIBA
- 11.12 Labcorp
- 11.13 Merck
- 11.14 OptiBio
- 11.15 RANDOX
- 11.16 Roche
- 11.17 SIEMENS Healthineers










