 |
市場調查報告書
商品編碼
1740919
赫賽汀市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Herceptin Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
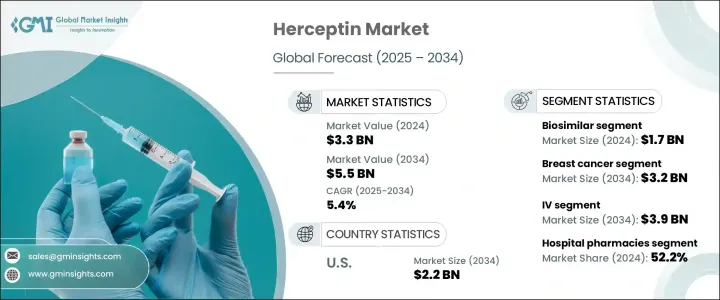
2024 年全球赫賽汀市場價值為 33 億美元,預計到 2034 年將以 5.4% 的複合年成長率成長至 55 億美元。赫賽汀,又稱曲妥珠單抗,仍是 HER2 陽性乳癌的基石療法,HER2 陽性乳癌是一種以 HER2 蛋白過度表現為特徵的亞型,可加速腫瘤生長。隨著乳癌繼續位列全球最常見癌症之一,對赫賽汀等標靶療法的需求正在急劇上升。對精準醫療的日益關注,以及全球晚期癌症治療可近性的擴大,正在顯著推動市場成長。此外,人們對早期癌症檢測認知的提高、篩檢計畫的改進以及持續的研發努力以改進標靶療法,共同推動了赫賽汀市場的蓬勃發展。醫療保健投資的增加、保險覆蓋範圍的擴大以及患者對生物製劑而非傳統化療的偏好日益成長,進一步支持了市場擴張。新興經濟體也不斷崛起,為赫賽汀及其生物相似藥創造了巨大的機會,特別是隨著法規核准速度的加速和各地區醫療保健服務的改善。
赫賽汀是一種單株抗體,能夠精準靶向並阻斷 HER2 蛋白,從而抑制癌細胞的生長和擴散。透過干擾 HER2 訊號傳導,赫賽汀可以減緩腫瘤進展並顯著提高存活率,尤其適用於 HER2 陽性乳癌患者。這種乳癌以侵襲性強且對常規療法反應不佳而聞名。對許多患者來說,赫賽汀如同一條生命線,在傳統療法無法發揮作用的情況下,帶來更佳的臨床療效。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 33億美元 |
| 預測值 | 55億美元 |
| 複合年成長率 | 5.4% |
赫賽汀市場中生物相似藥部分在2024年的營收為17億美元,預計到2034年將以5.6%的複合年成長率成長。具有同等臨床療效的生物相似藥正在使挽救生命的治療更容易獲得,尤其是在原研究生物製劑高昂成本限制患者獲得治療的地區。隨著多種曲妥珠單抗生物相似藥獲得批准並以全球品牌進入市場,預計可負擔的替代藥物供應將大幅增加,使全球更多患者受益。
赫賽汀市場主要分為乳癌和胃癌兩大類。乳癌在2024年佔據最大佔有率,貢獻了總收入的60%以上。乳癌發生率的上升,尤其是在女性族群中,持續推動了赫賽汀的需求,因為赫賽汀已被證實能夠延長HER2陽性患者的生存期並降低復發率。
預計到 2034 年,美國赫賽汀市場規模將達到 22 億美元,這得益於強大的醫療保健體系、高度的癌症意識以及標靶療法的大力採用。
塑造競爭格局的領導者包括雷迪博士實驗室 (Dr. Reddy's Laboratories)、安進 (Amgen)、AryoGen Pharmed、Biocon、Celltrion Healthcare、基因泰克 (Genentech)、邁蘭 (Mylan NV)、輝瑞 (Pfizer)、上海復宏漢靚 (Shanghai Biolius Biolius Biomando)。這些公司正大力投資研發,推出生物相似藥,拓展新興市場,並加強病患支援計劃,以提升治療效果和市場佔有率。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- HER2陽性乳癌發生率上升
- 提高對標靶癌症治療的認知
- 生物相似藥的採用日益增多
- 藥物輸送系統的技術進步
- 產業陷阱與挑戰
- 與治療相關的副作用
- 嚴格的監管情景
- 成長動力
- 成長潛力分析
- 監管格局
- 川普政府關稅
- 對貿易的影響
- 貿易量中斷
- 報復措施
- 對產業的影響
- 供給側影響(原料)
- 主要材料價格波動
- 供應鏈重組
- 生產成本影響
- 需求面影響(售價)
- 價格傳導至終端市場
- 市佔率動態
- 消費者反應模式
- 供給側影響(原料)
- 受影響的主要公司
- 策略產業反應
- 供應鏈重組
- 定價和產品策略
- 政策參與
- 展望與未來考慮
- 對貿易的影響
- 技術格局
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按產品,2021 年至 2034 年
- 主要趨勢
- 生物製劑
- 生物相似藥
第6章:市場估計與預測:按應用,2021 年至 2034 年
- 主要趨勢
- 乳癌
- 胃癌
第7章:市場估計與預測:依管理路線,2021 年至 2034 年
- 主要趨勢
- 靜脈
- 皮下
第8章:市場估計與預測:按配銷通路,2021 年至 2034 年
- 主要趨勢
- 醫院藥房
- 專業藥房
- 網路藥局
第9章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- Amgen
- AryoGen Pharmed
- Biocon
- Celltrion Healthcare
- Dr. Reddy's Laboratories
- Genentech
- Mylan NV
- Pfizer
- Samsung Bioepis
- Sandoz
- Shanghai Henlius Biotech
The Global Herceptin Market was valued at USD 3.3 billion in 2024 and is estimated to grow at a CAGR of 5.4% to reach USD 5.5 billion by 2034. Herceptin, or trastuzumab, remains a cornerstone therapy for HER2-positive breast cancer, a subtype marked by the overexpression of the HER2 protein that accelerates tumor growth. As breast cancer continues to rank among the most commonly diagnosed cancers worldwide, the demand for targeted therapies like Herceptin is rising sharply. The increasing focus on precision medicine, along with expanding access to advanced cancer care globally, is significantly boosting market growth. Moreover, the heightened awareness around early cancer detection, improved screening programs, and continuous R&D efforts to refine targeted therapies are collectively driving momentum in the Herceptin landscape. Rising healthcare investments, expanding insurance coverage, and growing patient preference for biologics over traditional chemotherapy are further supporting market expansion. Emerging economies are also stepping up, creating vast opportunities for Herceptin and its biosimilars, particularly as regulatory approvals become faster and healthcare access improves across regions.

Herceptin, a monoclonal antibody, precisely targets and blocks the HER2 protein, disrupting the cancer cell's ability to grow and spread. By interfering with HER2 signaling, Herceptin slows tumor progression and significantly improves survival rates, particularly in HER2-positive breast cancer, which is known for its aggressive behavior and poor response to conventional therapies. For many patients, Herceptin offers a lifeline, delivering better clinical outcomes where traditional treatments fall short.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.3 Billion |
| Forecast Value | $5.5 Billion |
| CAGR | 5.4% |
The biosimilars segment of the Herceptin market accounted for USD 1.7 billion in revenue in 2024 and is estimated to grow at a CAGR of 5.6% through 2034. Biosimilars with comparable clinical efficacy are making life-saving treatments more accessible, especially in regions where the high cost of original biologics limits patient access. As multiple trastuzumab biosimilars gain approval and enter the market under global brands, the availability of affordable alternatives is expected to rise substantially, benefiting a larger pool of patients worldwide.
The Herceptin market is primarily divided between breast cancer and gastric cancer applications. Breast cancer held the largest share in 2024, generating over 60% of total revenue. The rising prevalence of breast cancer, especially among women, continues to drive demand for Herceptin, given its proven ability to extend survival and reduce recurrence rates for HER2-positive patients.
The U.S. Herceptin Market is forecasted to reach USD 2.2 billion by 2034, supported by a robust healthcare system, high levels of cancer awareness, and strong adoption of targeted therapies.
Leading players shaping the competitive landscape include Dr. Reddy's Laboratories, Amgen, AryoGen Pharmed, Biocon, Celltrion Healthcare, Genentech, Mylan N.V., Pfizer, Shanghai Henlius Biotech, Samsung Bioepis, and Sandoz. These companies are investing heavily in research, launching biosimilars, expanding into emerging markets, and strengthening patient support initiatives to enhance treatment outcomes and market presence.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising incidence of HER2-positive breast cancer
- 3.2.1.2 Increasing awareness of targeted cancer therapies
- 3.2.1.3 Growing adoption of biosimilars
- 3.2.1.4 Technological advancement in drug delivery system
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Side effects associated with treatment
- 3.2.2.2 Stringent regulatory scenario
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the Industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Technological landscape
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Biologic
- 5.3 Biosimilars
Chapter 6 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Breast cancer
- 6.3 Stomach/gastric cancer
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Intravenous
- 7.3 Subcutaneous
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals pharmacies
- 8.3 Specialty pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Amgen
- 10.2 AryoGen Pharmed
- 10.3 Biocon
- 10.4 Celltrion Healthcare
- 10.5 Dr. Reddy’s Laboratories
- 10.6 Genentech
- 10.7 Mylan N.V.
- 10.8 Pfizer
- 10.9 Samsung Bioepis
- 10.10 Sandoz
- 10.11 Shanghai Henlius Biotech






