 |
市場調查報告書
商品編碼
1721607
體外診斷市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測In-vitro Diagnostics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
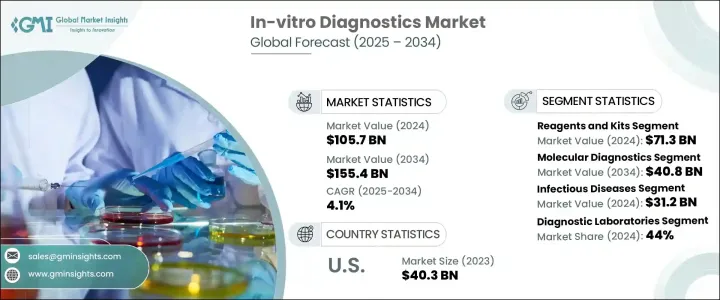
2024 年全球體外診斷市場價值為 1,057 億美元,預計到 2034 年將以 4.1% 的複合年成長率成長,達到 1,554 億美元。體外診斷 (IVD) 包括用於分析人體外部血液、尿液和組織等生物樣本的各種醫療設備、試劑和系統。這些工具在檢測、診斷和管理疾病方面發揮著至關重要的作用,可以做出更快、更準確的臨床決策。隨著世界各地的醫療保健系統不斷向預防保健和精準醫療轉變,對先進診斷工具的需求正在穩步上升。
IVD 解決方案不僅在醫院和臨床實驗室中得到越來越廣泛的應用,而且在家庭護理和照護端環境中也得到越來越廣泛的應用。慢性病和傳染病負擔的不斷加重、人們對早期疾病檢測認知的不斷提高以及新型診斷技術的出現共同推動了這個市場的擴張。此外,人工智慧和數位健康平台在診斷中的應用正在改變結果的生成、解釋和傳遞方式,為個人化和即時護理創造了新的途徑。支持性的監管政策、有利的報銷方案以及已開發和新興經濟體對醫療數位化的強力推動進一步加速了該產業的成長軌跡。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 1057億美元 |
| 預測值 | 1554億美元 |
| 複合年成長率 | 4.1% |
試劑和試劑盒領域在 2024 年創造了 713 億美元的產值,預計在 2025 年至 2034 年期間的複合年成長率將達到 4.4%。這一成長主要得益於對精準和早期診斷日益成長的需求,以及對客製化治療方案日益成長的需求。試劑和試劑盒涵蓋廣泛的產品,包括生化試劑、抗體、檢測試劑盒、探針和引物,所有這些都是運行各種診斷測試所必需的。隨著分子診斷技術的進步,這些產品現在能夠提供比傳統檢測方法更準確、更快速的結果,使其成為現代診斷實驗室中不可或缺的一部分。
分子診斷領域在 2024 年佔了 25.5% 的市場佔有率,預計將大幅成長,到 2034 年達到 408 億美元。該領域受益於不斷提高測試準確性、週轉時間和易用性的創新。分子診斷工具由於能夠在分子層面上識別病原體和基因突變,現已成為檢測傳染病、遺傳疾病和癌症的首選。與通常需要幾天才能返回結果的傳統測試不同,分子診斷可以在幾小時甚至幾分鐘內產生可操作的見解,從而能夠更快地做出治療決策並改善患者的治療效果。
2024 年,北美體外診斷市場規模達到 451 億美元,憑藉強大的醫療基礎設施、較高的慢性病負擔以及早期採用先進技術,保持主導地位。儘管監管環境嚴格,該地區仍繼續支持尖端診斷解決方案的批准和商業化。
全球體外診斷市場的主要參與者包括雅培實驗室、ACON 實驗室、安捷倫科技、碧迪公司、Bio-Rad 實驗室、Dragerwerk、羅氏公司、丹納赫集團、美敦力、Meridian Bioscience、Nova Biomedical、珀金埃爾默、西門子醫療和 Sysmex 公司。這些公司專注於研發、策略合作夥伴關係和全球擴張,以滿足對先進診斷技術日益成長的需求。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 全球傳染病和慢性病發病率不斷上升
- 個人化醫療的採用率不斷提高,需求不斷成長
- 發展中國家擴大採用即時檢測
- 配備先進診斷儀器的病理實驗室和服務數量激增
- 產業陷阱與挑戰
- 診斷服務費用高昂
- 不利的報銷情況
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按產品,2021 - 2034 年
- 主要趨勢
- 試劑和試劑盒
- 儀器
第6章:市場估計與預測:按測試類型,2021 - 2034 年
- 主要趨勢
- 臨床化學
- 免疫測定/免疫化學
- 分子診斷
- 血液學
- 尿液分析
- 其他測試類型
第7章:市場估計與預測:按應用,2021 - 2034 年
- 主要趨勢
- 腫瘤學
- 傳染病
- 糖尿病
- 心臟病學
- 腎臟病學
- 自體免疫疾病
- 藥物檢測/藥物基因組學
- 其他應用
第8章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 醫院
- 診斷實驗室
- 學術和研究機構
- 其他最終用途
第9章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- Abbott Laboratories
- ACON Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Biomerieux
- Bio-Rad Laboratories
- Dragerwerk
- F. Hoffmann-La Roche
- Danaher Corporation
- Medtronic
- Meridian Bioscience
- Nova Biomedical
- PerkinElmer
- Siemens Healthineers
- Sysmex Corporation
The Global In-Vitro Diagnostics Market was valued at USD 105.7 billion in 2024 and is estimated to grow at a CAGR of 4.1% to reach USD 155.4 billion by 2034. In-vitro diagnostics (IVD) includes a wide range of medical devices, reagents, and systems used to analyze biological samples such as blood, urine, and tissues outside the human body. These tools play a vital role in detecting, diagnosing, and managing diseases, enabling faster and more accurate clinical decisions. As healthcare systems around the world continue to shift toward preventive care and precision medicine, the demand for advanced diagnostic tools is rising steadily.

IVD solutions are increasingly being adopted not only in hospitals and clinical laboratories but also in home care and point-of-care settings. The growing burden of chronic and infectious diseases, rising awareness about early disease detection, and the emergence of novel diagnostic technologies are collectively driving the expansion of this market. Moreover, the adoption of artificial intelligence and digital health platforms in diagnostics is transforming the way results are generated, interpreted, and delivered, creating new avenues for personalized and real-time care. Supportive regulatory policies, favorable reimbursement scenarios, and a strong push for healthcare digitization across developed and emerging economies further accelerate the industry's growth trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $105.7 Billion |
| Forecast Value | $155.4 Billion |
| CAGR | 4.1% |
The reagents and kits segment generated USD 71.3 billion in 2024 and is expected to grow at a CAGR of 4.4% from 2025 to 2034. This growth is largely driven by the increasing need for precise and early-stage diagnosis, as well as the rising demand for customized treatment plans. Reagents and kits cover a broad spectrum of products, including biochemical reagents, antibodies, assay kits, probes, and primers, all of which are essential for running a variety of diagnostic tests. With the advancement of molecular diagnostics, these products are now capable of delivering highly accurate and faster results compared to traditional testing methods, making them indispensable in modern diagnostic labs.
The molecular diagnostics segment held a 25.5% market share in 2024 and is expected to grow significantly, reaching USD 40.8 billion by 2034. This segment benefits from ongoing innovations that enhance test accuracy, turnaround time, and ease of use. Molecular diagnostic tools are now the preferred option for detecting infectious diseases, genetic disorders, and cancer, thanks to their ability to identify pathogens and genetic mutations at a molecular level. Unlike conventional tests that often require days to return results, molecular diagnostics can produce actionable insights within hours or even minutes, enabling faster treatment decisions and better patient outcomes.
The North America In-Vitro Diagnostics Market generated USD 45.1 billion in 2024, maintaining a dominant position due to strong healthcare infrastructure, a high burden of chronic illnesses, and early adoption of advanced technologies. Despite a stringent regulatory landscape, the region continues to support the approval and commercialization of cutting-edge diagnostic solutions.
Key players in the Global In-Vitro Diagnostics Market include Abbott Laboratories, ACON Laboratories, Agilent Technologies, Becton, Dickinson and Company, Bio-Rad Laboratories, Dragerwerk, F. Hoffmann-La Roche, Danaher Corporation, Medtronic, Meridian Bioscience, Nova Biomedical, PerkinElmer, Siemens Healthineers, and Sysmex Corporation. These companies focus on research and development, strategic partnerships, and global expansion to meet the rising demand for advanced diagnostic technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of infectious and chronic diseases worldwide
- 3.2.1.2 Growing adoption and rising demand for personalized medicine
- 3.2.1.3 Increasing adoption of point-of-care testing in developing countries
- 3.2.1.4 Surging number of pathology labs and services equipped with advanced diagnostics machine
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of diagnostic services
- 3.2.2.2 Unfavorable reimbursement scenario
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Reagents and kits
- 5.3 Instruments
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Clinical chemistry
- 6.3 Immunoassay/immunochemistry
- 6.4 Molecular diagnostics
- 6.5 Hematology
- 6.6 Urinalysis
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Infectious diseases
- 7.4 Diabetes
- 7.5 Cardiology
- 7.6 Nephrology
- 7.7 Autoimmune diseases
- 7.8 Drug testing/pharmacogenomics
- 7.9 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic laboratories
- 8.4 Academic and research institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 ACON Laboratories
- 10.3 Agilent Technologies
- 10.4 Becton, Dickinson and Company
- 10.5 Biomerieux
- 10.6 Bio-Rad Laboratories
- 10.7 Dragerwerk
- 10.8 F. Hoffmann-La Roche
- 10.9 Danaher Corporation
- 10.10 Medtronic
- 10.11 Meridian Bioscience
- 10.12 Nova Biomedical
- 10.13 PerkinElmer
- 10.14 Siemens Healthineers
- 10.15 Sysmex Corporation









