 |
市場調查報告書
商品編碼
1708185
淋巴瘤治療市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Lymphoma Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球淋巴瘤治療市場規模達 83 億美元,預估 2025 年至 2034 年複合年成長率為 8.4%。淋巴瘤是一種影響淋巴系統的癌症,由淋巴球(一種白血球)的異常增生所引起。它主要表現在淋巴結中,也可能影響其他組織。淋巴瘤主要有兩種類型:非何杰金氏淋巴瘤和霍奇金淋巴瘤,以霍奇金淋巴瘤中 Reed-Sternberg 細胞的存在為特徵。症狀通常包括淋巴結腫大、發燒、疲勞和食慾不振。霍奇金淋巴瘤的負擔仍然很高,尤其是在年輕族群中,而一些地區醫療基礎設施不足導致存活率較低。這種差異強調了改進治療方法的必要性,特別是在低收入地區。
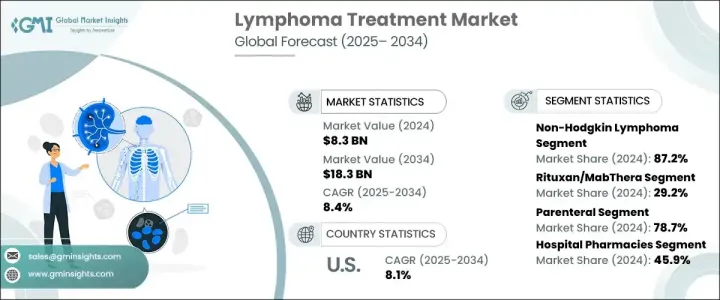
非何杰金氏淋巴瘤 (NHL) 因其盛行率較高且亞型多樣而佔據市場主導地位,導致全球患者數量較大。發病率的上升繼續推動市場的成長。利妥昔單抗等單株抗體療法的使用徹底改變了淋巴瘤的治療。利妥昔單抗針對 B 淋巴細胞上的 CD20 分子,已被證明可有效治療 NHL 和結節性淋巴細胞為主的霍奇金淋巴瘤 (NLPHL)。該療法提高了反應率,延長了緩解時間,提高了存活率,鞏固了其作為淋巴瘤治療基石的地位。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 83億美元 |
| 預測值 | 183億美元 |
| 複合年成長率 | 8.4% |
2024 年,腸外給藥佔了 78.7% 的市場佔有率,凸顯了其主導地位。這種方法是將藥物直接注射到血液中,確保藥物快速吸收,使其成為需要立即干涉的惡性淋巴瘤的理想選擇。腸外途徑可實現精確給藥和控制輸送,這對於免疫療法、CAR-T 細胞療法和高劑量化療等先進療法至關重要。由於嚴重症狀或腸道問題而無法耐受口服藥物的患者可以從這種方法中受益。此外,對於口服治療效果不佳的復發性或難治性淋巴瘤病例,腸外給藥仍是首選方法。
2024 年,醫院藥局佔據最大的市場佔有率,為 45.9%,這得益於化療、免疫療法和 CAR T 細胞療法等醫院管理療法的廣泛使用。這些專門的治療需要精心的準備和監測,最好在醫院環境中進行。醫院藥房仍然是這些定製藥物和高成本生物製劑的主要來源。接受專科腫瘤治療的醫院入院人數不斷成長,進一步鞏固了該領域的巨大市場佔有率。
在英國,淋巴瘤治療市場預計將在 2025 年至 2034 年間持續成長。該地區淋巴瘤患病率的不斷上升推動了對創新治療方案的需求。精準醫療、免疫療法和 CAR T 細胞療法的進步正在滿足這一需求。此外,生命科學產業策略和英國脫歐推動的權力下放等措施正在加強英國創新和生產國內治療方法的能力,從而促進該地區的整體市場成長。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 淋巴瘤盛行率不斷上升
- 治療方案的進步
- 新型標靶療法的核准率不斷提高
- 產業陷阱與挑戰
- 治療費用高
- 延遲診斷
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按類型,2021 年至 2034 年
- 主要趨勢
- 霍奇金淋巴瘤
- 非何杰金氏淋巴瘤
第6章:市場估計與預測:依藥物類型,2021 年至 2034 年
- 主要趨勢
- 利妥昔單抗/美羅華
- 來那度胺
- 依魯替尼
- 阿德塞特里斯
- 可瑞達
- 奧狄沃
- 其他藥物類型
第7章:市場估計與預測:依管理路線,2021 年至 2034 年
- 主要趨勢
- 口服
- 腸外
第8章:市場估計與預測:按配銷通路,2021 年至 2034 年
- 主要趨勢
- 醫院藥房
- 零售藥局
- 網路藥局
第9章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第10章:公司簡介
- AstraZeneca
- Bayer
- Biogen
- BioGene
- Bristol-Myers Squibb
- Celgene
- Eli Lilly and Company
- F. Hoffmann-La Roche
- Gilead Sciences
- Incyte
- Johnson & Johnson
- Juno Therapeutics
- Merck
- Novartis
- Seattle Genetics
- Takeda Pharmaceutical
The Global Lymphoma Treatment Market reached USD 8.3 billion in 2024 and is projected to exhibit a CAGR of 8.4% from 2025 to 2034. Lymphoma, a type of cancer affecting the lymphatic system, arises from abnormal proliferation of lymphocytes, a form of white blood cell. It primarily manifests in lymph nodes and can also affect other tissues. There are two primary types non-Hodgkin lymphoma and Hodgkin lymphoma-distinguished by the presence of Reed-Sternberg cells in Hodgkin lymphoma. Symptoms often include lymphadenopathy, fever, fatigue, and appetite loss. The burden of Hodgkin lymphoma remains high, especially among young populations, while inadequate healthcare infrastructure in some regions contributes to lower survival rates. This disparity emphasizes the need for improved treatment approaches, particularly in low-income regions.

Non-Hodgkin lymphoma (NHL) dominates the market due to its higher prevalence and multiple subtypes, resulting in a larger patient population worldwide. Increasing incidence rates continue to propel the market's growth. The use of monoclonal antibody therapies, such as Rituximab, has revolutionized lymphoma treatment. Rituximab targets the CD20 molecule on B lymphocytes and has proven effective in managing both NHL and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL). The therapy enhances response rates, prolongs remission duration, and boosts survival rates, reinforcing its position as a cornerstone in lymphoma treatment.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $8.3 Billion |
| Forecast Value | $18.3 Billion |
| CAGR | 8.4% |
Parenteral administration accounted for 78.7% of the market share in 2024, underscoring its dominance. This method, which involves injecting medication directly into the bloodstream, ensures rapid drug absorption, making it ideal for aggressive lymphomas requiring immediate intervention. Parenteral routes allow for precise dosing and controlled delivery, which is critical for advanced therapies such as immunotherapy, CAR T-cell therapy, and high-dose chemotherapy. Patients who are unable to tolerate oral medications due to severe symptoms or bowel issues benefit from this approach. Furthermore, parenteral administration remains the preferred method for relapsed or refractory lymphoma cases that do not respond well to oral treatments.
Hospital pharmacies held the largest market share at 45.9% in 2024, driven by the widespread use of hospital-administered therapies such as chemotherapy, immunotherapy, and CAR T-cell therapies. These specialized treatments require careful preparation and monitoring, which is best handled within a hospital setting. Hospital pharmacies remain the primary source for these tailored medications and high-cost biologics. The growing volume of hospital admissions for specialized oncology care further reinforces the segment's substantial market share.
In the United Kingdom, the lymphoma treatment market is expected to witness consistent growth between 2025 and 2034. The increasing prevalence of lymphoma in the region is driving demand for innovative treatment solutions. Advancements in precision medicine, immunotherapy, and CAR T-cell therapies are meeting this demand. Additionally, initiatives such as the Life Sciences Industrial Strategy and Brexit-driven devolution efforts are strengthening the UK's capacity to innovate and produce domestic treatments, boosting the region's overall market growth.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of lymphoma
- 3.2.1.2 Advancements in treatment options
- 3.2.1.3 Growing approval of novel targeted therapies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High treatment costs
- 3.2.2.2 Delayed diagnosis
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Hodgkin lymphoma
- 5.3 Non-Hodgkin lymphoma
Chapter 6 Market Estimates and Forecast, By Drug Type, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Rituxan/MabThera
- 6.3 Revlimid
- 6.4 Imbruvica
- 6.5 Adcetris
- 6.6 Keytruda
- 6.7 Opdivo
- 6.8 Other drug types
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oral
- 7.3 Parenteral
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 AstraZeneca
- 10.2 Bayer
- 10.3 Biogen
- 10.4 BioGene
- 10.5 Bristol-Myers Squibb
- 10.6 Celgene
- 10.7 Eli Lilly and Company
- 10.8 F. Hoffmann-La Roche
- 10.9 Gilead Sciences
- 10.10 Incyte
- 10.11 Johnson & Johnson
- 10.12 Juno Therapeutics
- 10.13 Merck
- 10.14 Novartis
- 10.15 Seattle Genetics
- 10.16 Takeda Pharmaceutical










