 |
市場調查報告書
商品編碼
1699293
胎兒監護市場機會、成長動力、產業趨勢分析及 2025-2034 年預測Fetal Monitoring Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034 |
||||||
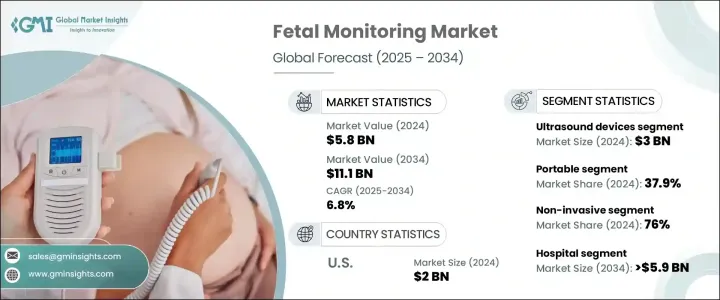
2024 年全球胎兒監護市場規模達到 58 億美元,預計 2025 年至 2034 年複合年成長率為 6.8%,這得益於對先進產婦保健解決方案的需求不斷成長。人們對妊娠相關併發症的認知不斷提高以及早期發現胎兒窘迫的需求是推動市場成長的關鍵因素。隨著醫療保健提供者強調積極主動的產婦和胎兒護理,創新監測技術的採用正在加速。
醫療技術的不斷進步正在改變胎兒監護,使其更加精確、高效和方便。增強的感測器精確度、即時監控能力以及人工智慧驅動的分析正在透過早期診斷和介入徹底改變產前護理。這些創新不僅改善了懷孕結果,也降低了併發症的風險,確保了更好的整體孕產婦和胎兒健康。遠端監控和遠距醫療解決方案的日益整合進一步提高了可近性,特別是在醫療保健基礎設施有限的地區。隨著數位醫療的普及,醫療保健提供者正在利用數據驅動的洞察力來提供個人化和及時的干涉。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 58億美元 |
| 預測值 | 111億美元 |
| 複合年成長率 | 6.8% |
便攜性仍然是市場區隔的關鍵因素,將設備分為攜帶式和非攜帶式兩類。 2024年,攜帶式胎兒監護設備由於其便利性和移動性將佔據37.9%的市場。預計到 2034 年,該領域的複合年成長率將達到 6.9%,反映出人們對家庭和門診胎兒監護的偏好日益成長。攜帶式設備能夠持續監測,無需頻繁去醫院,從而增強了醫療專業人員和準媽媽的產前護理管理。因此,這些解決方案簡化了產婦保健服務,同時減輕了醫院設施的負擔。
依方法細分,凸顯了非侵入性胎兒監測相較於傳統侵入性技術的日益受到青睞。 2024 年,非侵入式解決方案佔據市場主導地位,佔有 76% 的佔有率,預計該領域將在未來幾年見證最高成長。非侵入性技術提供準確的胎兒監測,同時消除與侵入性方法相關的風險,例如感染和出血。對安全、高效、舒適的監測解決方案的需求不斷成長,這加強了向非侵入性選擇的轉變,尤其是在發達的醫療保健市場。
美國仍然是全球胎兒監護產業的主導者,2024 年的收入將達到 20 億美元。妊娠相關併發症發生率的增加是推動全國需求的主要因素。隨著醫療保健系統注重風險管理和早期干預,先進的胎兒監護解決方案的採用不斷擴大。人工智慧診斷和無線監控的整合進一步加強了市場成長,提高了患者護理標準,同時最佳化了醫療資源。隨著不斷創新和支持性監管框架的出現,美國胎兒監護市場將在未來十年持續擴張。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 妊娠相關併發症發生率不斷上升
- 胎兒監測設備的技術進步
- 政府不斷推出旨在促進母嬰健康的舉措
- 非侵入式設備的採用激增
- 早產數量增加,不孕症治療採用率上升
- 產業陷阱與挑戰
- 設備成本高
- 翻新產品的可用性
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 未來市場趨勢
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 戰略展望
第5章:市場估計與預測:按產品,2021 年至 2034 年
- 主要趨勢
- 超音波設備
- 2D超音波
- 3D和4D超音波
- 多普勒成像
- 電子胎兒監視器(EFM)
- 外部 EFM
- 內部 EFM
- 子宮收縮監測儀
- 配件和耗材
- 其他產品
第6章:市場估計與預測:按便攜性,2021 年至 2034 年
- 主要趨勢
- 便攜的
- 非攜帶式
第7章:市場估計與預測:依方法,2021 年至 2034 年
- 主要趨勢
- 侵入性
- 非侵入性
第8章:市場估計與預測:依最終用途,2021 年至 2034 年
- 主要趨勢
- 醫院
- 專科診所
- 其他最終用途
第9章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- Becton, Dickinson and Company
- Biolight Medical
- Cardinal Health
- DOTO Health
- EDAN Instrument
- FUJIFILM Holdings Corporation
- GE Healthcare
- Huntleigh Healthcare (Arjo)
- Koninklijke Philips
- MedGyn products
- Medtronic
- Natus Medical
- Neoventa Medical
- Siemens Healthineers
- Spacelabs Healthcare
The Global Fetal Monitoring Market reached USD 5.8 billion in 2024 and is projected to grow at a CAGR of 6.8% from 2025 to 2034, driven by the increasing demand for advanced maternal healthcare solutions. Rising awareness of pregnancy-related complications and the need for early detection of fetal distress are key factors propelling market growth. As healthcare providers emphasize proactive maternal and fetal care, the adoption of innovative monitoring technologies is accelerating.

Continuous advancements in medical technology are transforming fetal monitoring, making it more precise, efficient, and accessible. Enhanced sensor accuracy, real-time monitoring capabilities, and AI-driven analytics are revolutionizing prenatal care by enabling early diagnosis and intervention. These innovations not only improve pregnancy outcomes but also reduce the risk of complications, ensuring better overall maternal and fetal health. The growing integration of remote monitoring and telehealth solutions further enhances accessibility, particularly in regions with limited healthcare infrastructure. As digital health adoption rises, healthcare providers are leveraging data-driven insights to deliver personalized and timely interventions.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $5.8 Billion |
| Forecast Value | $11.1 Billion |
| CAGR | 6.8% |
Portability remains a crucial factor in market segmentation, distinguishing devices into portable and non-portable categories. In 2024, portable fetal monitoring devices accounted for 37.9% of the market share, driven by their convenience and mobility. This segment is projected to grow at a CAGR of 6.9% through 2034, reflecting a rising preference for home-based and outpatient fetal monitoring. Portable devices enable continuous monitoring without frequent hospital visits, enhancing prenatal care management for both healthcare professionals and expectant mothers. As a result, these solutions are streamlining maternal healthcare services while reducing the burden on hospital facilities.
Segmentation by method highlights the increasing preference for non-invasive fetal monitoring over traditional invasive techniques. In 2024, non-invasive solutions dominated the market with a 76% share, and this segment is expected to witness the highest growth in the coming years. Non-invasive technologies offer accurate fetal monitoring while eliminating risks associated with invasive methods, such as infections and bleeding. The growing demand for safe, efficient, and comfortable monitoring solutions is reinforcing the shift toward non-invasive options, particularly in developed healthcare markets.
The United States remains a dominant player in the global fetal monitoring industry, generating USD 2 billion in revenue in 2024. The increasing incidence of pregnancy-related complications is a primary factor driving demand across the country. As healthcare systems focus on risk management and early intervention, the adoption of advanced fetal monitoring solutions continues to expand. The integration of AI-powered diagnostics and wireless monitoring further strengthens market growth, enhancing patient care standards while optimizing healthcare resources. With continuous innovation and supportive regulatory frameworks, the US fetal monitoring market is poised for sustained expansion in the coming decade.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing incidence of pregnancy-related complications
- 3.2.1.2 Technological advancements in fetal monitoring devices
- 3.2.1.3 Rising government initiatives aimed at promoting maternal and child health
- 3.2.1.4 Surge in adoption of non-invasive devices
- 3.2.1.5 Increasing number of preterm births and rising adoption of infertility treatment
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of devices
- 3.2.2.2 Availability of refurbished products
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Ultrasound devices
- 5.2.1 2D ultrasound
- 5.2.2 3D and 4D ultrasound
- 5.2.3 Doppler imaging
- 5.3 Electronic fetal monitoring (EFM)
- 5.3.1 External EFM
- 5.3.2 Internal EFM
- 5.4 Uterine contraction monitor
- 5.5 Accessories and consumables
- 5.6 Other products
Chapter 6 Market Estimates and Forecast, By Portability, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Portable
- 6.3 Non-portable
Chapter 7 Market Estimates and Forecast, By Method, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Invasive
- 7.3 Non-invasive
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Specialty clinics
- 8.4 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Becton, Dickinson and Company
- 10.2 Biolight Medical
- 10.3 Cardinal Health
- 10.4 DOTO Health
- 10.5 EDAN Instrument
- 10.6 FUJIFILM Holdings Corporation
- 10.7 GE Healthcare
- 10.8 Huntleigh Healthcare (Arjo)
- 10.9 Koninklijke Philips
- 10.10 MedGyn products
- 10.11 Medtronic
- 10.12 Natus Medical
- 10.13 Neoventa Medical
- 10.14 Siemens Healthineers
- 10.15 Spacelabs Healthcare












