 |
市場調查報告書
商品編碼
1665106
監管資訊管理系統市場機會、成長動力、產業趨勢分析與預測 2025 - 2034Regulatory Information Management System Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球監管資訊管理系統( RIMS) 市場價值為 22 億美元,預計 2025 年至 2034 年期間將以 10.9% 的強勁複合年成長率成長。隨著全球市場聯繫日益緊密,法規發展速度也比以往任何時候都快,企業被迫採用先進的解決方案來幫助它們始終領先於合規要求。對準確性、透明度和營運效率的需求從未如此重要,因此,組織正在轉向尖端的 RIMS 來簡化流程、增強資料處理並即時保持法規遵循。預計各行各業,特別是製藥、醫療保健和製造業,將越來越依賴這些技術來應對複雜的監管環境。
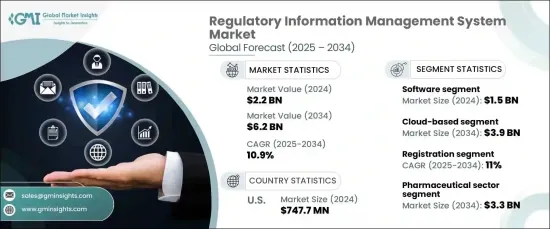
市場分為軟體和服務,其中軟體領域處於領先地位。 2024 年,軟體部門創造的收入為 15 億美元,預計在預測期內將顯著成長。軟體解決方案佔據主導地位,因為它們能夠簡化監管流程、最大限度地減少人工錯誤並加強合規管理。這些工具旨在隨著業務成長而擴展,使其成為各種規模公司的理想選擇。隨著監管需求變得越來越動態和複雜,軟體解決方案可協助組織簡化提交、追蹤合規更新並減少營運效率低下。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 22億美元 |
| 預測值 | 62億美元 |
| 複合年成長率 | 10.9% |
在部署方面,市場分為基於雲端的解決方案和內部部署的解決方案。基於雲端的系統已獲得廣泛關注,預計到 2034 年該領域的規模將達到 39 億美元。這些系統在監管環境中尤其有價值,因為企業需要即時更新和無縫整合以保持靈活性和合規性。透過利用雲端技術,公司可以確保掌握不斷變化的法規,同時最大限度地降低合規失敗的風險。
從地區來看,2024 年美國監管資訊管理系統市場價值為 7.477 億美元。美國仍然是製藥和醫療器材等行業的中心,高效處理監管資料、提交和批准至關重要。隨著這些行業的公司尋求在多個司法管轄區保持合規的方法,美國越來越廣泛地採用 RIMS 解決方案的趨勢繼續推動市場成長。
目錄
第 1 章:方法論與範圍
第 2 章:執行摘要
第 3 章:產業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 監管要求日益複雜
- 監管資料量不斷增加
- 技術進步
- 臨床試驗和藥物核准的成長
- 產業陷阱與挑戰
- 實施成本高
- 成長動力
- 成長潛力分析
- 監管格局
- 美國
- 歐洲
- 技術格局
- 未來市場趨勢
- 定價分析
- 差距分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第 5 章:市場估計與預測:按組件,2021 年至 2034 年
- 主要趨勢
- 軟體
- 服務
第 6 章:市場估計與預測:按部署類型,2021 年至 2034 年
- 主要趨勢
- 基於雲端
- 本地
第 7 章:市場估計與預測:按應用,2021 年至 2034 年
- 主要趨勢
- 登記
- 提交
- 出版
- 其他應用
第 8 章:市場估計與預測:依最終用途,2021 年至 2034 年
- 主要趨勢
- 製藥業
- 醫療器材領域
- 其他最終用戶
第 9 章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- AmpleLogic
- ArisGlobal
- Calyx
- Dassault Systemes
- DDi
- DXC Technology
- Ennov
- Ithos Global (Cordance Group)
- Kalypso (Rockwell Automation)
- Korber
- LORENZ Life Sciences Group
- MasterControl
- PhlexGlobal
- Rimsys
- Veeva Systems
The Global Regulatory Information Management System (RIMS) Market was valued at USD 2.2 billion in 2024 and is forecasted to grow at a robust CAGR of 10.9% from 2025 to 2034. This growth is largely driven by the increasing complexity of regulatory frameworks, the rising volume of compliance data, and the pressure on organizations to adopt more efficient management systems. With global markets becoming more interconnected and regulations evolving faster than ever, businesses are being compelled to adopt advanced solutions that help them stay ahead of compliance requirements. The need for accuracy, transparency, and operational efficiency has never been more critical, and as a result, organizations are turning to cutting-edge RIMS to streamline processes, enhance data handling, and maintain regulatory compliance in real-time. Industries across the board, especially those dealing with pharmaceuticals, healthcare, and manufacturing, are expected to increasingly rely on these technologies to navigate the intricate regulatory landscape.

The market is segmented into software and services, with the software segment leading the charge. In 2024, the software segment generated USD 1.5 billion in revenue and is expected to see significant growth over the forecast period. Software solutions dominate due to their ability to streamline regulatory processes, minimize manual errors, and enhance compliance management. These tools are designed to scale with business growth, making them ideal for companies of various sizes. As regulatory demands grow more dynamic and complex, software solutions help organizations simplify submissions, track compliance updates, and reduce operational inefficiencies.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.2 Billion |
| Forecast Value | $6.2 Billion |
| CAGR | 10.9% |
In terms of deployment, the market is divided into cloud-based and on-premises solutions. Cloud-based systems have gained substantial traction, with the sector projected to reach USD 3.9 billion by 2034. The popularity of cloud-based solutions stems from their scalability, cost-effectiveness, and the flexibility they offer for remote access and collaboration. These systems are particularly valuable in a regulatory environment where businesses need real-time updates and seamless integration to maintain agility and compliance. By leveraging cloud technologies, companies can ensure they stay on top of constantly changing regulations while minimizing the risk of compliance failures.
Regionally, the U.S. regulatory information management system market was valued at USD 747.7 million in 2024. The country's stringent regulatory environment, driven by federal agencies enforcing rigorous compliance standards, has led to an increased demand for advanced management systems. The U.S. remains a hub for industries such as pharmaceuticals and medical devices, where the efficient handling of regulatory data, submissions, and approvals is critical. This trend toward greater adoption of RIMS solutions in the U.S. continues to fuel market growth as companies in these sectors look for ways to stay compliant across multiple jurisdictions.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing complexity of regulatory requirements
- 3.2.1.2 Rising volume of regulatory data
- 3.2.1.3 Technological advancements
- 3.2.1.4 Growth in clinical trials and drug approvals
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High implementation cost
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Pricing analysis
- 3.8 Gap analysis
- 3.9 Porter’s analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Component, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Software
- 5.3 Services
Chapter 6 Market Estimates and Forecast, By Deployment Type, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Cloud-based
- 6.3 On-premises
Chapter 7 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Registration
- 7.3 Submission
- 7.4 Publishing
- 7.5 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pharmaceutical sector
- 8.3 Medical device sector
- 8.4 Other end-users
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 AmpleLogic
- 10.2 ArisGlobal
- 10.3 Calyx
- 10.4 Dassault Systemes
- 10.5 DDi
- 10.6 DXC Technology
- 10.7 Ennov
- 10.8 Ithos Global (Cordance Group)
- 10.9 Kalypso (Rockwell Automation)
- 10.10 Korber
- 10.11 LORENZ Life Sciences Group
- 10.12 MasterControl
- 10.13 PhlexGlobal
- 10.14 Rimsys
- 10.15 Veeva Systems





